Page 23 - Read Online
P. 23
Page 16 of 35 Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40
Table 2. psaAWH related properties of some typical adsorbents
Adsorption process Desorption process
Type Materials Water uptake (RH), Desorption temperature, water release, Ref.
adsorption time desorption time
-1
-1
Classic adsorbents Silica 0.4 g g (75%), - 100 °C, ~0.4 g g , - [40]
-1
-1
SAPO-34 0.3 g g (40%), 80 min 120 °C, 0.3 g g , 10 min [91]
-1
-1
Zeolite 13X 0.3 g g (40%), 60 min 120 °C, 0.2 g g , 20 min [91]
-1
-1
MOFs MOF-801 0.2 g g (30%), 200 min 85 °C, 0.2 g g , 30 min [34]
-1
-1
MOF-303 0.4 g g (40%), 80 min 85 °C, 0.4 g g , 40 min [91]
-1
-1
Al-fumarate 0.4 g g (40%), 150 min 65 °C, 0.4 g g , 20 min [91]
-1
-1
MOF-303/333 0.4 g g (40%), - 85 °C, 0.4 g g , one adsorption/desorption cycle [79]
75 min
-1
-1
Cu-AD-SA MOF 0.15 g g (20%), 160 min 85 °C, 0.15 g g , - [92]
-1
-1
MIL-101 (Cr)@LiCl 0.77 g g (30%), 240 min 83 °C, 0.6 g g , 90 min [83]
-1
-1
COFs COF-432 0.23 g g (40%), - 35 °C, 0.23 g g , - [27]
-1
-1
COF-480-hydrazide 0.35 g g (40%), - 85 °C, 0.33 g g , - [110]
-1
-1
Hygroscopic PNIPAAM/Alg IPN gel 0.89 g g (90%), - 50 °C, 0.7 g g , - [37]
polymers
-1
-1
Super moisture-absorbent gel 2 g g (75%), 50 min Sunlight, 2 g g , 10 min [116]
(SMAG)
-1
-1
ZnO hydrogel ~0.6 g g (90%), 45 min Sunlight, 0.6 g g , 15 min [114]
-2
-2
POG 6 kg m (90%), 12 h Sunlight, 5.5 kg m , 700 min [122]
-1
-1
SHPFs ~0.6 g g (30%), 30 min 60 °C, ~0.6 g g , 30 min [152]
-1
-1
CPOSs CPOS-6 0.23 g g (70%), 52 min 40 °C, 0.12 g g , 30 min [29]
-1
-1
Porous carbon Steam-80 0.21 g g (26%), 45 min Sunlight, 0.16 g g , 15 min [151]
COFs: Covalent organic frameworks; CPOSs: crystalline porous organic salts; MOFs: metal-organic frameworks; POG: photothermal organogel;
RH: relative humidity; SHPFs: super hygroscopic polymer films.
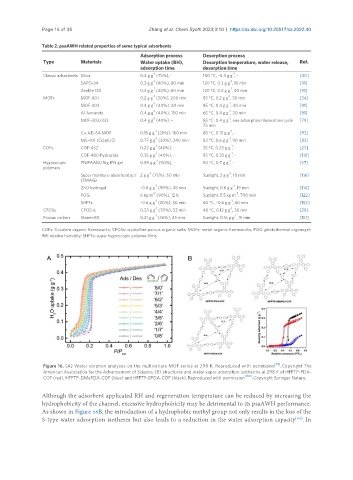
Figure 16. (A) Water sorption analyses on the multivariate MOF series at 298 K. Reproduced with permission [79] . Copyright The
American Association for the Advancement of Science; (B) structures and water vapor adsorption isotherms at 298 K of HFPTP-PDA-
COF (red), HFPTP-DMePDA-COF (blue) and HFPTP-BPDA-COF (black). Reproduced with permission [100] . Copyright Springer Nature.
Although the adsorbent applicated RH and regeneration temperature can be reduced by increasing the
hydrophobicity of the channel, excessive hydrophobicity may be detrimental to its psaAWH performance.
As shown in Figure 16B, the introduction of a hydrophobic methyl group not only results in the loss of the
S-type water adsorption isotherm but also leads to a reduction in the water adsorption capacity . In
[100]