Page 18 - Read Online
P. 18
Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40 Page 11 of 35
Figure 9. Nucleation site of water cluster in three COFs pore. Reproduced with permission [100] . Copyright Springer Nature.
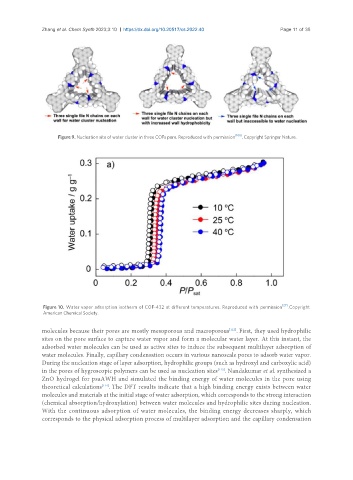
Figure 10. Water vapor adsorption isotherm of COF-432 at different temperatures. Reproduced with permission [27] . Copyright
American Chemical Society.
molecules because their pores are mostly mesoporous and macroporous . First, they used hydrophilic
[112]
sites on the pore surface to capture water vapor and form a molecular water layer. At this instant, the
adsorbed water molecules can be used as active sites to induce the subsequent multilayer adsorption of
water molecules. Finally, capillary condensation occurs in various nanoscale pores to adsorb water vapor.
During the nucleation stage of layer adsorption, hydrophilic groups (such as hydroxyl and carboxylic acid)
in the pores of hygroscopic polymers can be used as nucleation sites . Nandakumar et al. synthesized a
[113]
ZnO hydrogel for psaAWH and simulated the binding energy of water molecules in the pore using
theoretical calculations . The DFT results indicate that a high binding energy exists between water
[114]
molecules and materials at the initial stage of water adsorption, which corresponds to the strong interaction
(chemical absorption/hydroxylation) between water molecules and hydrophilic sites during nucleation.
With the continuous adsorption of water molecules, the binding energy decreases sharply, which
corresponds to the physical adsorption process of multilayer adsorption and the capillary condensation