Page 14 - Read Online
P. 14
Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40 Page 7 of 35
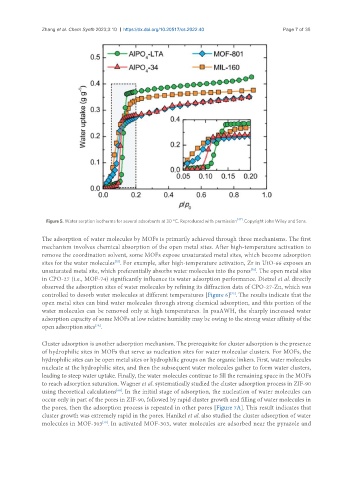
Figure 5. Water sorption isotherms for several adsorbents at 30 °C. Reproduced with permission [47] . Copyright John Wiley and Sons.
The adsorption of water molecules by MOFs is primarily achieved through three mechanisms. The first
mechanism involves chemical absorption of the open metal sites. After high-temperature activation to
remove the coordination solvent, some MOFs expose unsaturated metal sites, which become adsorption
sites for the water molecules . For example, after high-temperature activation, Zr in UiO-66 exposes an
[75]
unsaturated metal site, which preferentially absorbs water molecules into the pores . The open metal sites
[76]
in CPO-27 (i.e., MOF-74) significantly influence its water adsorption performance. Dietzel et al. directly
observed the adsorption sites of water molecules by refining its diffraction data of CPO-27-Zn, which was
controlled to desorb water molecules at different temperatures [Figure 6] . The results indicate that the
[77]
open metal sites can bind water molecules through strong chemical adsorption, and this portion of the
water molecules can be removed only at high temperatures. In psaAWH, the sharply increased water
adsorption capacity of some MOFs at low relative humidity may be owing to the strong water affinity of the
open adsorption sites .
[78]
Cluster adsorption is another adsorption mechanism. The prerequisite for cluster adsorption is the presence
of hydrophilic sites in MOFs that serve as nucleation sites for water molecular clusters. For MOFs, the
hydrophilic sites can be open metal sites or hydrophilic groups on the organic linkers. First, water molecules
nucleate at the hydrophilic sites, and then the subsequent water molecules gather to form water clusters,
leading to steep water uptake. Finally, the water molecules continue to fill the remaining space in the MOFs
to reach adsorption saturation. Wagner et al. systematically studied the cluster adsorption process in ZIF-90
using theoretical calculations . In the initial stage of adsorption, the nucleation of water molecules can
[68]
occur only in part of the pores in ZIF-90, followed by rapid cluster growth and filling of water molecules in
the pores, then the adsorption process is repeated in other pores [Figure 7A]. This result indicates that
cluster growth was extremely rapid in the pores. Hanikel et al. also studied the cluster adsorption of water
molecules in MOF-303 . In activated MOF-303, water molecules are adsorbed near the pyrazole and
[79]