Page 11 - Read Online
P. 11
Page 4 of 35 Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40
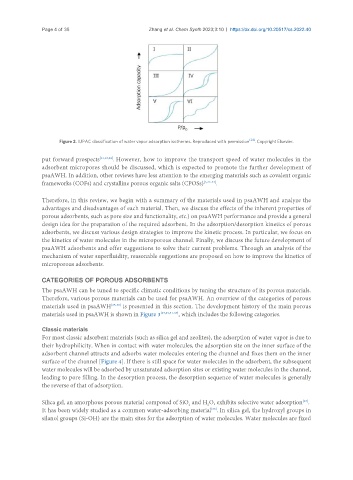
Figure 2. IUPAC classification of water vapor adsorption isotherms. Reproduced with permission [24] . Copyright Elsevier.
put forward prospects [13,16,22] . However, how to improve the transport speed of water molecules in the
adsorbent micropores should be discussed, which is expected to promote the further development of
psaAWH. In addition, other reviews have less attention to the emerging materials such as covalent organic
frameworks (COFs) and crystalline porous organic salts (CPOSs) [5,22-24] .
Therefore, in this review, we begin with a summary of the materials used in psaAWH and analyze the
advantages and disadvantages of each material. Then, we discuss the effects of the inherent properties of
porous adsorbents, such as pore size and functionality, etc.) on psaAWH performance and provide a general
design idea for the preparation of the required adsorbent. In the adsorption/desorption kinetics of porous
adsorbents, we discuss various design strategies to improve the kinetic process. In particular, we focus on
the kinetics of water molecules in the microporous channel. Finally, we discuss the future development of
psaAWH adsorbents and offer suggestions to solve their current problems. Through an analysis of the
mechanism of water superfluidity, reasonable suggestions are proposed on how to improve the kinetics of
microporous adsorbents.
CATEGORIES OF POROUS ADSORBENTS
The psaAWH can be tuned to specific climatic conditions by tuning the structure of its porous materials.
Therefore, various porous materials can be used for psaAWH. An overview of the categories of porous
materials used in psaAWH [25-30] is presented in this section. The development history of the main porous
materials used in psaAWH is shown in Figure 3 [27,29,31-37] , which includes the following categories.
Classic materials
For most classic adsorbent materials (such as silica gel and zeolites), the adsorption of water vapor is due to
their hydrophilicity. When in contact with water molecules, the adsorption site on the inner surface of the
adsorbent channel attracts and adsorbs water molecules entering the channel and fixes them on the inner
surface of the channel [Figure 4]. If there is still space for water molecules in the adsorbent, the subsequent
water molecules will be adsorbed by unsaturated adsorption sites or existing water molecules in the channel,
leading to pore filling. In the desorption process, the desorption sequence of water molecules is generally
the reverse of that of adsorption.
Silica gel, an amorphous porous material composed of SiO and H O, exhibits selective water adsorption .
[23]
2
2
It has been widely studied as a common water-adsorbing material . In silica gel, the hydroxyl groups in
[38]
silanol groups (Si-OH) are the main sites for the adsorption of water molecules. Water molecules are fixed