Page 10 - Read Online
P. 10
Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40 Page 3 of 35
Table 1. Summary of characteristics of several freshwater production technologies
Technology name Geographic dependencies Weather dependencies Implementation premise
Seawater desalination Yes No Coastal
Rainwater collection No Yes Rainfall
Fog collection No Yes Foggy
Condensation No Yes Difficult to implement at low RH
psaAWH No No Presence of atmospheric humidity
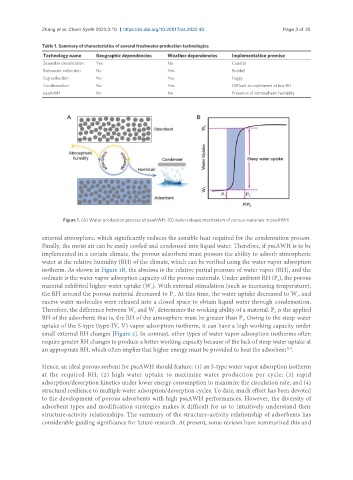
Figure 1. (A) Water production process of psaAWH; (B) water release mechanism of porous materials in psaAWH.
external atmosphere, which significantly reduces the sensible heat required for the condensation process.
Finally, the moist air can be easily cooled and condensed into liquid water. Therefore, if psaAWH is to be
implemented in a certain climate, the porous adsorbent must possess the ability to adsorb atmospheric
water at the relative humidity (RH) of the climate, which can be verified using the water vapor adsorption
isotherm. As shown in Figure 1B, the abscissa is the relative partial pressure of water vapor (RH), and the
ordinate is the water vapor adsorption capacity of the porous materials. Under ambient RH (P ), the porous
2
material exhibited higher water uptake (W ). With external stimulation (such as increasing temperature),
2
the RH around the porous material decreased to P . At this time, the water uptake decreased to W , and
1
1
excess water molecules were released into a closed space to obtain liquid water through condensation.
Therefore, the difference between W and W determines the working ability of a material. P is the applied
2
1
2
RH of the adsorbent; that is, the RH of the atmosphere must be greater than P . Owing to the steep water
2
uptake of the S-type (type-IV, V) vapor adsorption isotherm, it can have a high working capacity under
small external RH changes [Figure 2]. In contrast, other types of water vapor adsorption isotherms often
require greater RH changes to produce a better working capacity because of the lack of steep water uptake at
an appropriate RH, which often implies that higher energy must be provided to heat the adsorbent .
[15]
Hence, an ideal porous sorbent for psaAWH should feature: (1) an S-type water vapor adsorption isotherm
at the required RH; (2) high water uptake to maximize water production per cycle; (3) rapid
adsorption/desorption kinetics under lower energy consumption to maximize the circulation rate; and (4)
structural resilience to multiple water adsorption/desorption cycles. To date, much effort has been devoted
to the development of porous adsorbents with high psaAWH performances. However, the diversity of
adsorbent types and modification strategies makes it difficult for us to intuitively understand their
structure-activity relationships. The summary of the structure-activity relationship of adsorbents has
considerable guiding significance for future research. At present, some reviews have summarized this and