Page 12 - Read Online
P. 12
Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40 Page 5 of 35
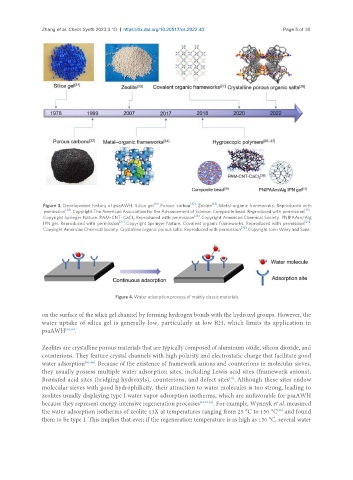
Figure 3. Development history of psaAWH. Silica gel [31] . Porous carbon [32] . Zeolite [33] . Metal-organic frameworks. Reproduced with
permission [34] . Copyright The American Association for the Advancement of Science. Composite bead. Reproduced with permission [35] .
Copyright Springer Nature. PAM-CNT-CaCl . Reproduced with permission [36] . Copyright American Chemical Society. PNIPAAm/Alg
2
IPN gel. Reproduced with permission [37] . Copyright Springer Nature. Covalent organic frameworks. Reproduced with permission [27] .
Copyright American Chemical Society. Crystalline organic porous salts. Reproduced with permission [29] . Copyright John Wiley and Sons.
Figure 4. Water adsorption process of mainly classic materials.
on the surface of the silica gel channel by forming hydrogen bonds with the hydroxyl groups. However, the
water uptake of silica gel is generally low, particularly at low RH, which limits its application in
psaAWH [39,40] .
Zeolites are crystalline porous materials that are typically composed of aluminum oxide, silicon dioxide, and
counterions. They feature crystal channels with high polarity and electrostatic charge that facilitate good
water adsorption [41-44] . Because of the existence of framework anions and counterions in molecular sieves,
they usually possess multiple water adsorption sites, including Lewis acid sites (framework anions),
Brønsted acid sites (bridging hydroxyls), counterions, and defect sites . Although these sites endow
[41]
molecular sieves with good hydrophilicity, their attraction to water molecules is too strong, leading to
zeolites usually displaying type I water vapor adsorption isotherms, which are unfavorable for psaAWH
because they represent energy-intensive regeneration processes [23,45,46] . For example, Wynnyk et al. measured
the water adsorption isotherms of zeolite 13X at temperatures ranging from 25 °C to 150 °C and found
[45]
them to be type I. This implies that even if the regeneration temperature is as high as 150 °C, several water