Page 21 - Read Online
P. 21
Page 14 of 35 Zhang et al. Chem Synth 2023;3:10 https://dx.doi.org/10.20517/cs.2022.40
Figure 13. Schematic of water adsorption on CPOS-6 featuring a double hydrogen bond system under different humidity conditions.
Reproduced with permission [29] . Copyright John Wiley and Sons.
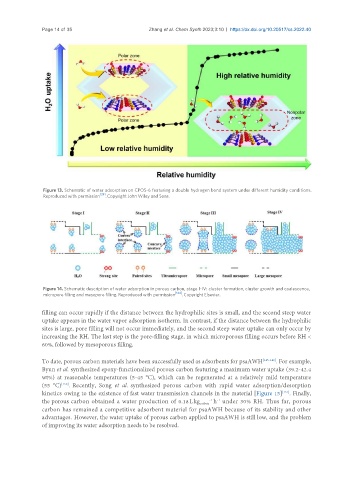
Figure 14. Schematic description of water adsorption in porous carbon, stage I-IV: cluster formation, cluster growth and coalescence,
micropore filling and mesopore filling. Reproduced with permission [144] . Copyright Elsevier.
filling can occur rapidly if the distance between the hydrophilic sites is small, and the second steep water
uptake appears in the water vapor adsorption isotherm. In contrast, if the distance between the hydrophilic
sites is large, pore filling will not occur immediately, and the second steep water uptake can only occur by
increasing the RH. The last step is the pore-filling stage, in which microporous filling occurs before RH <
60%, followed by mesoporous filling.
To date, porous carbon materials have been successfully used as adsorbents for psaAWH [145-149] . For example,
Byun et al. synthesized epoxy-functionalized porous carbon featuring a maximum water uptake (39.2-42.4
wt%) at reasonable temperatures (5-45 °C), which can be regenerated at a relatively mild temperature
(55 °C) . Recently, Song et al. synthesized porous carbon with rapid water adsorption/desorption
[150]
kinetics owing to the existence of fast water transmission channels in the material [Figure 15] . Finally,
[151]
the porous carbon obtained a water production of 0.18 L kg carbon -1 h under 30% RH. Thus far, porous
-1
carbon has remained a competitive adsorbent material for psaAWH because of its stability and other
advantages. However, the water uptake of porous carbon applied to psaAWH is still low, and the problem
of improving its water adsorption needs to be resolved.