Page 29 - Read Online
P. 29
Laubach et al. Cancer Drug Resist 2023;6:611-41 https://dx.doi.org/10.20517/cdr.2023.60 Page 631
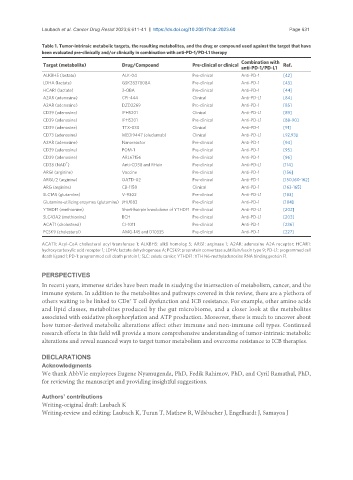
Table 1. Tumor-intrinsic metabolic targets, the resulting metabolites, and the drug or compound used against the target that have
been evaluated pre-clinically and/or clinically in combination with anti-PD-1/PD-L1 therapy
Combination with
Target (metabolite) Drug/Compound Pre-clinical or clinical Ref.
anti-PD-1/PD-L1
ALKBH5 (lactate) ALK-04 Pre-clinical Anti-PD-1 [42]
LDHA (lactate) GSK2837808A Pre-clinical Anti-PD-1 [43]
HCAR1 (lactate) 3-OBA Pre-clinical Anti-PD-1 [44]
A2AR (adenosine) CPI-444 Clinical Anti-PD-L1 [84]
A2AR (adenosine) DZD2269 Pre-clinical Anti-PD-1 [85]
CD39 (adenosine) IPH5201 Clinical Anti-PD-L1 [89]
CD39 (adenosine) IPH5201 Pre-clinical Anti-PD-L1 [88-90]
CD39 (adenosine) TTX-030 Clinical Anti-PD-1 [91]
CD73 (adenosine) MEDI9447 (oleclumab) Clinical Anti-PD-L1 [92,93]
A2AR (adenosine) Nanoreactor Pre-clinical Anti-PD-1 [94]
CD39 (adenosine) POM-1 Pre-clinical Anti-PD-1 [95]
CD39 (adenosine) ARL67156 Pre-clinical Anti-PD-1 [96]
+
CD38 (NAD ) Anti-CD38 and RHein Pre-clinical Anti-PD-L1 [114]
ARG1 (arginine) Vaccine Pre-clinical Anti-PD-1 [156]
ARG1/2 (arginine) OATD-02 Pre-clinical Anti-PD-1 [150,160-162]
ARG (arginine) CB-1158 Clinical Anti-PD-1 [163-165]
SLC1A5 (glutamine) V-9302 Pre-clinical Anti-PD-L1 [183]
Glutamine-utilizing enzymes (glutamine) JHU083 Pre-clinical Anti-PD-1 [184]
YTHDF1 (methionine) Short-hairpin knockdown of YTHDF1 Pre-clinical Anti-PD-L1 [202]
SLC43A2 (methionine) BCH Pre-clinical Anti-PD-L1 [203]
ACAT1 (cholesterol) CI-1011 Pre-clinical Anti-PD-1 [236]
PCSK9 (cholesterol) AMG-145 and D10335 Pre-clinical Anti-PD-1 [227]
ACAT1: Acyl-CoA cholesterol acyl transferase 1; ALKBH5: alkB homolog 5; ARG1: arginase 1; A2AR: adenosine A2A receptor; HCAR1:
hydroxycarboxylic acid receptor 1; LDHA: lactate dehydrogenase A; PCSK9: proprotein convertase subtilisin/kexin type 9; PD-L1: programmed cell
death ligand 1; PD-1: programmed cell death protein 1; SLC: solute carrier; YTHDF1: YTH N6-methyladenosine RNA binding protein F1.
PERSPECTIVES
In recent years, immense strides have been made in studying the intersection of metabolism, cancer, and the
immune system. In addition to the metabolites and pathways covered in this review, there are a plethora of
others waiting to be linked to CD8 T cell dysfunction and ICB resistance. For example, other amino acids
+
and lipid classes, metabolites produced by the gut microbiome, and a closer look at the metabolites
associated with oxidative phosphorylation and ATP production. Moreover, there is much to uncover about
how tumor-derived metabolic alterations affect other immune and non-immune cell types. Continued
research efforts in this field will provide a more comprehensive understanding of tumor-intrinsic metabolic
alterations and reveal nuanced ways to target tumor metabolism and overcome resistance to ICB therapies.
DECLARATIONS
Acknowledgments
We thank AbbVie employees Eugene Nyamugenda, PhD, Fedik Rahimov, PhD, and Cyril Ramathal, PhD,
for reviewing the manuscript and providing insightful suggestions.
Authors’ contributions
Writing-original draft: Laubach K
Writing-review and editing: Laubach K, Turan T, Mathew R, Wilsbacher J, Engelhardt J, Samayoa J