Page 24 - Read Online
P. 24
Page 626 Laubach et al. Cancer Drug Resist 2023;6:611-41 https://dx.doi.org/10.20517/cdr.2023.60
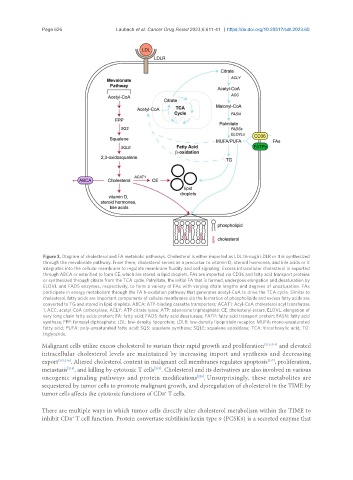
Figure 3. Diagram of cholesterol and FA metabolic pathways. Cholesterol is either imported as LDL through LDLR or it is synthesized
through the mevalonate pathway. From there, cholesterol serves as a precursor to vitamin D, steroid hormones, and bile acids or it
integrates into the cellular membrane to regulate membrane fluidity and cell signaling. Excess intracellular cholesterol is exported
through ABCA or esterified to form CE, which are stored in lipid droplets. FAs are imported via CD36 and fatty acid transport proteins
or synthesized through citrate from the TCA cycle. Palmitate, the initial FA that is formed, undergoes elongation and desaturation by
ELOVL and FADS enzymes, respectively, to form a variety of FAs with varying chain lengths and degrees of unsaturation. FAs
participate in energy metabolism through the FA b-oxidation pathway that generates acetyl-CoA to drive the TCA cycle. Similar to
cholesterol, fatty acids are important components of cellular membranes via the formation of phospholipids and excess fatty acids are
converted to TG and stored in lipid droplets. ABCA: ATP-binding cassette transporters; ACAT1: Acyl-CoA cholesterol acyl transferase
1; ACC: acetyl-CoA carboxylase; ACLY: ATP citrate lyase; ATP: adenosine triphosphate; CE: cholesteryl esters; ELOVL: elongation of
very long chain fatty acids protein; FA: fatty acid; FADS: fatty acid desaturase; FATP: fatty acid transport protein; FASN: fatty acid
synthase; FPP: farnesyl diphosphate; LDL: low-density lipoprotein; LDLR: low-density lipoprotein receptor; MUFA: mono-unsaturated
fatty acid; PUFA: poly-unsaturated fatty acid; SQS: squalene synthase; SQLE: squalene epoxidase; TCA: tricarboxylic acid; TG:
triglyceride.
Malignant cells utilize excess cholesterol to sustain their rapid growth and proliferation [212-214] and elevated
intracellular cholesterol levels are maintained by increasing import and synthesis and decreasing
export [215,216] . Altered cholesterol content in malignant cell membranes regulates apoptosis , proliferation,
[217]
[219]
[218]
metastasis , and killing by cytotoxic T cells . Cholesterol and its derivatives are also involved in various
oncogenic signaling pathways and protein modifications . Unsurprisingly, these metabolites are
[220]
sequestered by tumor cells to promote malignant growth, and dysregulation of cholesterol in the TIME by
+
tumor cells affects the cytotoxic functions of CD8 T cells.
There are multiple ways in which tumor cells directly alter cholesterol metabolism within the TIME to
inhibit CD8 T cell function. Protein convertase subtilisin/kexin type 9 (PCSK9) is a secreted enzyme that
+