Page 60 - Read Online
P. 60
Liu et al. Soft Sci 2024;4:44 https://dx.doi.org/10.20517/ss.2024.59 Page 3 of 21
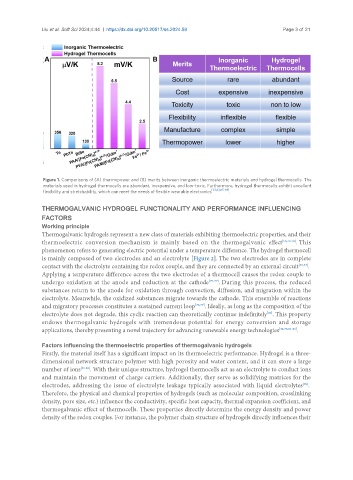
Figure 1. Comparisons of (A) thermopower and (B) merits between inorganic thermoelectric materials and hydrogel thermocells. The
materials used in hydrogel thermocells are abundant, inexpensive, and low-toxic. Furthermore, hydrogel thermocells exhibit excellent
flexibility and stretchability, which can meet the needs of flexible wearable electronics [33,63,65-69] .
THERMOGALVANIC HYDROGEL FUNCTIONALITY AND PERFORMANCE INFLUENCING
FACTORS
Working principle
Thermogalvanic hydrogels represent a new class of materials exhibiting thermoelectric properties, and their
thermoelectric conversion mechanism is mainly based on the thermogalvanic effect [56,70-72] . This
phenomenon refers to generating electric potential under a temperature difference. The hydrogel thermocell
is mainly composed of two electrodes and an electrolyte [Figure 2]. The two electrodes are in complete
contact with the electrolyte containing the redox couple, and they are connected by an external circuit [73,74] .
Applying a temperature difference across the two electrodes of a thermocell causes the redox couple to
undergo oxidation at the anode and reduction at the cathode [75-77] . During this process, the reduced
substances return to the anode for oxidation through convection, diffusion, and migration within the
electrolyte. Meanwhile, the oxidized substances migrate towards the cathode. This ensemble of reactions
and migratory processes constitutes a sustained current loop [78,79] . Ideally, as long as the composition of the
electrolyte does not degrade, this cyclic reaction can theoretically continue indefinitely . This property
[80]
endows thermogalvanic hydrogels with tremendous potential for energy conversion and storage
applications, thereby presenting a novel trajectory for advancing renewable energy technologies [32,78,81-86] .
Factors influencing the thermoelectric properties of thermogalvanic hydrogels
Firstly, the material itself has a significant impact on its thermoelectric performance. Hydrogel is a three-
dimensional network structure polymer with high porosity and water content, and it can store a large
number of ions [87,88] . With their unique structure, hydrogel thermocells act as an electrolyte to conduct ions
and maintain the movement of charge carriers. Additionally, they serve as solidifying matrices for the
electrodes, addressing the issue of electrolyte leakage typically associated with liquid electrolytes .
[89]
Therefore, the physical and chemical properties of hydrogels (such as molecular composition, crosslinking
density, pore size, etc.) influence the conductivity, specific heat capacity, thermal expansion coefficient, and
thermogalvanic effect of thermocells. These properties directly determine the energy density and power
density of the redox couples. For instance, the polymer chain structure of hydrogels directly influences their