Page 61 - Read Online
P. 61
Page 4 of 21 Liu et al. Soft Sci 2024;4:44 https://dx.doi.org/10.20517/ss.2024.59
3-
4-
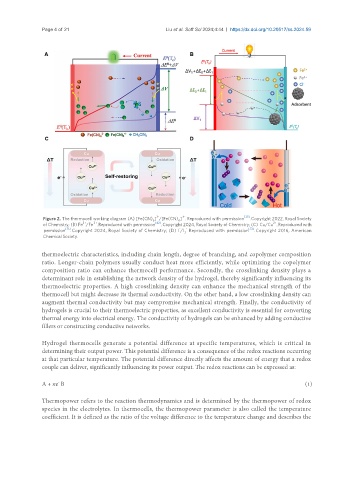
Figure 2. The thermocell working diagram (A) [Fe(CN) ] /[Fe(CN) ] . Reproduced with permission [33] . Copyright 2022, Royal Society
6
6
2+
2+
3+
of Chemistry; (B) Fe /Fe . Reproduced with permission [42] . Copyright 2024, Royal Society of Chemistry; (C) Cu/Cu . Reproduced with
-
-
permission [71] . Copyright 2024, Royal Society of Chemistry; (D) I /I Reproduced with permission [72] . Copyright 2016, American
3
Chemical Society.
thermoelectric characteristics, including chain length, degree of branching, and copolymer composition
ratio. Longer-chain polymers usually conduct heat more efficiently, while optimizing the copolymer
composition ratio can enhance thermocell performance. Secondly, the crosslinking density plays a
determinant role in establishing the network density of the hydrogel, thereby significantly influencing its
thermoelectric properties. A high crosslinking density can enhance the mechanical strength of the
thermocell but might decrease its thermal conductivity. On the other hand, a low crosslinking density can
augment thermal conductivity but may compromise mechanical strength. Finally, the conductivity of
hydrogels is crucial to their thermoelectric properties, as excellent conductivity is essential for converting
thermal energy into electrical energy. The conductivity of hydrogels can be enhanced by adding conductive
fillers or constructing conductive networks.
Hydrogel thermocells generate a potential difference at specific temperatures, which is critical in
determining their output power. This potential difference is a consequence of the redox reactions occurring
at that particular temperature. The potential difference directly affects the amount of energy that a redox
couple can deliver, significantly influencing its power output. The redox reactions can be expressed as:
A + ne B (1)
-
Thermopower refers to the reaction thermodynamics and is determined by the thermopower of redox
species in the electrolytes. In thermocells, the thermopower parameter is also called the temperature
coefficient. It is defined as the ratio of the voltage difference to the temperature change and describes the