Page 57 - Read Online
P. 57
Page 6 of 10 Zhu et al. Soft Sci 2024;4:21 https://dx.doi.org/10.20517/ss.2024.01
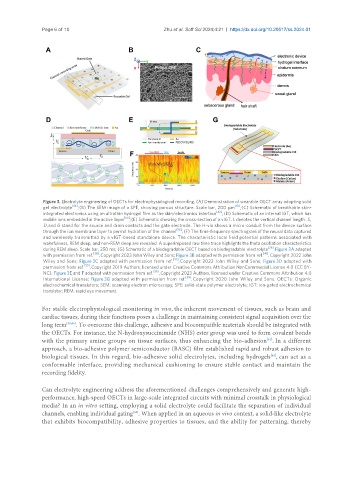
Figure 3. Electrolyte engineering of OECTs for electrophysiological recording. (A) Demonstration of wearable OECT array adopting solid
gel electrolyte [36] ; (B) The SEM image of a SPE, showing porous structure. Scale bar, 200 μm [41] ; (C) Schematic of breathable skin-
integrated electronics using an ultrathin hydrogel film as the skin/electronics interface [42] ; (D) Schematic of an internal IGT, which has
mobile ions embedded in the active layer [55] ; (E) Schematic showing the cross-section of a vIGT. L denotes the vertical channel length. S,
D, and G stand for the source and drain contacts and the gate electrode. The H-via shows a micro-conduit from the device surface
through the ion membrane layer to permit hydration of the channel [56] ; (F) The time-frequency spectrogram of the neural data captured
and wirelessly transmitted by a vIGT-based standalone device. The characteristic local field potential patterns associated with
wakefulness, REM sleep, and non-REM sleep are revealed. A superimposed raw time trace highlights the theta oscillation characteristics
during REM sleep. Scale bar, 250 ms; (G) Schematic of a biodegradable OECT based on biodegradable electrolyte [39] . Figure 3A adapted
with permission from ref. [36] , Copyright 2023 John Wiley and Sons; Figure 3B adapted with permission from ref. [41] , Copyright 2022 John
Wiley and Sons; Figure 3C adapted with permission from ref. [42] , Copyright 2022 John Wiley and Sons; Figure 3D adapted with
permission from ref. [55] , Copyright 2019 Authors, licensed under Creative Commons Attribution NonCommercial License 4.0 (CC BY-
NC); Figure 3E and F adapted with permission from ref. [56] , Copyright 2023 Authors, licensed under Creative Commons Attribution 4.0
International License; Figure 3G adapted with permission from ref. [39] , Copyright 2020 John Wiley and Sons. OECTs: Organic
electrochemical transistors; SEM: scanning electron microscopy; SPE: solid-state polymer electrolyte; IGT: ion-gated electrochemical
transistor; REM: rapid eye movement.
For stable electrophysiological monitoring in vivo, the inherent movement of tissues, such as brain and
cardiac tissues, during their functions poses a challenge in maintaining consistent signal acquisition over the
long term [59,60] . To overcome this challenge, adhesive and biocompatible materials should be integrated with
the OECTs. For instance, the N-hydroxysuccinimide (NHS) ester group was used to form covalent bonds
[61]
with the primary amine groups on tissue surfaces, thus enhancing the bio-adhesion . In a different
approach, a bio-adhesive polymer semiconductor (BASC) film established rapid and robust adhesion to
[42]
biological tissues. In this regard, bio-adhesive solid electrolytes, including hydrogels , can act as a
conformable interface, providing mechanical cushioning to ensure stable contact and maintain the
recording fidelity.
Can electrolyte engineering address the aforementioned challenges comprehensively and generate high-
performance, high-speed OECTs in large-scale integrated circuits with minimal crosstalk in physiological
media? In an in vitro setting, employing a solid electrolyte could facilitate the separation of individual
channels, enabling individual gating . When applied in an aqueous in vivo context, a solid-like electrolyte
[53]
that exhibits biocompatibility, adhesive properties to tissues, and the ability for patterning, thereby