Page 54 - Read Online
P. 54
Zhu et al. Soft Sci 2024;4:21 https://dx.doi.org/10.20517/ss.2024.01 Page 3 of 10
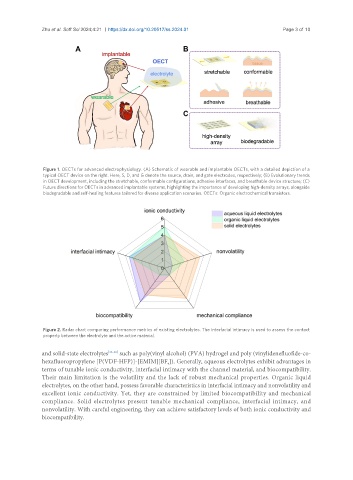
Figure 1. OECTs for advanced electrophysiology. (A) Schematic of wearable and implantable OECTs, with a detailed depiction of a
typical OECT device on the right. Here, S, D, and G denote the source, drain, and gate electrodes, respectively; (B) Evolutionary trends
in OECT development, including the stretchable, conformable configurations, adhesive interfaces, and breathable device structure; (C)
Future directions for OECTs in advanced implantable systems, highlighting the importance of developing high-density arrays, alongside
biodegradable and self-healing features tailored for diverse application scenarios. OECTs: Organic electrochemical transistors.
Figure 2. Radar chart comparing performance metrics of existing electrolytes. The interfacial intimacy is used to assess the contact
property between the electrolyte and the active material.
and solid-state electrolytes [35-39] such as poly(vinyl alcohol) (PVA) hydrogel and poly (vinylidenefluofide-co-
hexafluoropropylene [P(VDF-HFP)]-[EMIM][BF ]). Generally, aqueous electrolytes exhibit advantages in
4
terms of tunable ionic conductivity, interfacial intimacy with the channel material, and biocompatibility.
Their main limitation is the volatility and the lack of robust mechanical properties. Organic liquid
electrolytes, on the other hand, possess favorable characteristics in interfacial intimacy and nonvolatility and
excellent ionic conductivity. Yet, they are constrained by limited biocompatibility and mechanical
compliance. Solid electrolytes present tunable mechanical compliance, interfacial intimacy, and
nonvolatility. With careful engineering, they can achieve satisfactory levels of both ionic conductivity and
biocompatibility.