Page 77 - Read Online
P. 77
Luo et al. Soft Sci 2024;4:7 https://dx.doi.org/10.20517/ss.2023.40 Page 9 of 12
Table 1. Comparison of behavior of flexible sensors based on the ACO electrode with those of non-enzymatic glucose sensors based
on other reported materials
Materials Electrode fabrication Limit of Sensitivity Applied Flexibility Ref.
-1
detection µA·mM ·cm -2 potential (V)
( µM)
Si-CuO core-shell MPI method; E evaporator 740 2,324.09 1.0 (vs. × [40]
nanowire Ag/AgCl)
Cu O/TiO A JDF-05 benchtop machine at an applied 14.4 1.0366 0.1 (vs. × [41]
2 2
voltage of 21 kV and a propulsion rate of Hg/HgO)
1.0 mL/h; 600 °C
CuO 450 °C at atmospheric pressure; NH ; 650 °C 59 263 0.54 (n/A) × [42]
3
Cu/Cu O 700 °C 0.31 621.12 0.4 (n/A) × [43]
2
6
Au@CuO/V CT x HF aqueous, argon, vigorous stirring, low 5 1.124 × 10 0.45 (vs. SCE) × [44]
2
temperature
MXene/NiCo-LDH HF, vacuum, centrifugation, high temperature 0.53 67.75 0.45 (vs. SCE) × [45]
Ti C T -Cu O Centrifugation, vacuum, HF, high temperature 10 11.061 0.6 (vs. × [46]
2
3 2 x
Ag/AgCl)
Pt/MXene/CH/Pt HCl, high temperature, lithography 29.15 3.43 0.2 (vs. √ [47]
Ag/AgCl)
ACO Room temperature 1 0.87 -0.4 (vs. √ This
Ag/AgCl) work
ACO: Annealed Cu-Oxide; CH: conductive hydrogel; HF: hydrofluoric acid ; LDH: layered double hydroxide ; MPI: micro-propulsive injection; SCE:
saturated calomel electrode.
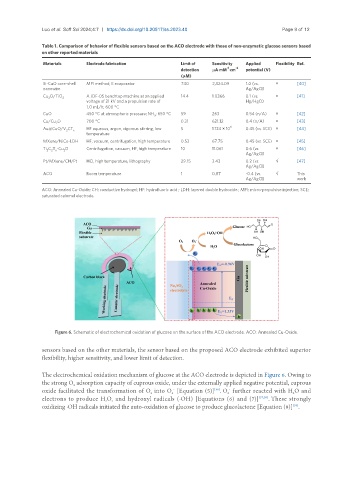
Figure 6. Schematic of electrochemical oxidation of glucose on the surface of the ACO electrode. ACO: Annealed Cu-Oxide.
sensors based on the other materials, the sensor based on the proposed ACO electrode exhibited superior
flexibility, higher sensitivity, and lower limit of detection.
The electrochemical oxidation mechanism of glucose at the ACO electrode is depicted in Figure 6. Owing to
the strong O adsorption capacity of cuprous oxide, under the externally applied negative potential, cuprous
2
oxide facilitated the transformation of O into O [Equation (5)] . O further reacted with H O and
[36]
-
-
2
2
2
2
electrons to produce H O and hydroxyl radicals (·OH) [Equations (6) and (7)] [37,38] . These strongly
2
2
[39]
oxidizing ·OH radicals initiated the auto-oxidation of glucose to produce glucolactone [Equation (8)] .