Page 76 - Read Online
P. 76
Page 8 of 12 Luo et al. Soft Sci 2024;4:7 https://dx.doi.org/10.20517/ss.2023.40
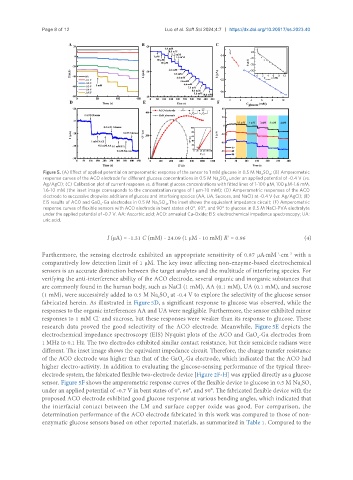
Figure 5. (A) Effect of applied potential on amperometric response of the sensor to 1 mM glucose in 0.5 M Na SO ; (B) Amperometric
2 4
response curves of the ACO electrode for different glucose concentrations in 0.5 M Na SO under an applied potential of -0.4 V (vs.
2 4
Ag/AgCl); (C) Calibration plot of current response vs. different glucose concentrations with fitted lines of 1-100 µM, 100 µM-1.6 mM,
1.6-10 mM (the inset image corresponds to the concentration ranges of 1 µm-10 mM); (D) Amperometric responses of the ACO
electrode to successive dropwise additions of glucose and interfering species (AA, UA, Sucrose, and NaCl) at -0.4 V (vs. Ag/AgCl); (E)
EIS results of ACO and GaO -Ga electrodes in 0.5 M Na SO . The inset shows the equivalent impedance circuit; (F) Amperometric
4
2
x
response curves of flexible sensors with ACO electrode in bent states of 0°, 60°, and 90° to glucose in 0.5 M NaCl-PVA electrolyte
under the applied potential of -0.7 V. AA: Ascorbic acid; ACO: annealed Cu-Oxide; EIS: electrochemical impedance spectroscopy; UA:
uric acid.
2
I (μA) = -1.31 C (mM) - 24.09 (1 μM - 10 mM) R = 0.96 (4)
Furthermore, the sensing electrode exhibited an appropriate sensitivity of 0.87 μA·mM ·cm with a
-2
-1
comparatively low detection limit of 1 μM. The key issue affecting non-enzyme-based electrochemical
sensors is an accurate distinction between the target analytes and the multitude of interfering species. For
verifying the anti-interference ability of the ACO electrode, several organic and inorganic substances that
are commonly found in the human body, such as NaCl (1 mM), AA (0.1 mM), UA (0.1 mM), and sucrose
(1 mM), were successively added to 0.5 M Na SO at -0.4 V to explore the selectivity of the glucose sensor
4
2
fabricated herein. As illustrated in Figure 5D, a significant response to glucose was observed, while the
responses to the organic interferences AA and UA were negligible. Furthermore, the sensor exhibited minor
-
responses to 1 mM Cl and sucrose, but these responses were weaker than its response to glucose. These
research data proved the good selectivity of the ACO electrode. Meanwhile, Figure 5E depicts the
electrochemical impedance spectroscopy (EIS) Nyquist plots of the ACO and GaO -Ga electrodes from
x
1 MHz to 0.1 Hz. The two electrodes exhibited similar contact resistance, but their semicircle radians were
different. The inset image shows the equivalent impedance circuit. Therefore, the charge transfer resistance
of the ACO electrode was higher than that of the GaO -Ga electrode, which indicated that the ACO had
x
higher electro-activity. In addition to evaluating the glucose-sensing performance of the typical three-
electrode system, the fabricated flexible two-electrode device [Figure 2F-H] was applied directly as a glucose
sensor. Figure 5F shows the amperometric response curves of the flexible device to glucose in 0.5 M Na SO 4
2
under an applied potential of -0.7 V in bent states of 0°, 60°, and 90°. The fabricated flexible device with the
proposed ACO electrode exhibited good glucose response at various bending angles, which indicated that
the interfacial contact between the LM and surface copper oxide was good. For comparison, the
determination performance of the ACO electrode fabricated in this work was compared to those of non-
enzymatic glucose sensors based on other reported materials, as summarized in Table 1. Compared to the