Page 75 - Read Online
P. 75
Page 10 of 32 Zhao et al. Soft Sci 2024;4:18 https://dx.doi.org/10.20517/ss.2024.04
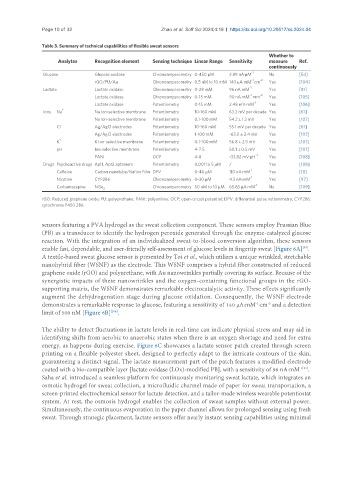
Table 3. Summary of technical capabilities of flexible sweat sensors
Whether to
Analytes Recognition element Sensing technique Linear Range Sensitivity measure Ref.
continuously
Glucose Glucose oxidase Chronoamperometry 0-450 μM 2.89 nA·μM -1 No [54]
-1 -2
rGO/PU/Au Chronoamperometry 0.5 nM to 10 mM 140 μA·mM ·cm Yes [104]
-1
Lactate Lactate oxidase Chronoamperometry 0-28 mM 96 nA·mM Yes [81]
-1
Lactate oxidase Chronoamperometry 0-15 mM 90 nA·mM ·mm -2 Yes [105]
-1
Lactate oxidase Potentiometry 0-15 mM 2.48 mV·mM Yes [106]
Ions Na + Na Ion-selective membrane Potentiometry 10-160 mM 63.2 mV per decade Yes [61]
Na Ion-selective membrane Potentiometry 0.1-100 mM 54.2 ± 1.3 mV Yes [107]
Cl - Ag/AgCl electrodes Potentiometry 10-160 mM 55.1 mV per decade Yes [61]
Ag/AgCl electrodes Potentiometry 1-100 mM -63.0 ± 2.4 mV Yes [107]
+
K K Ion-selective membrane Potentiometry 0.1-100 mM 56.8 ± 2.5 mV Yes [107]
pH Ion-selective membrane Potentiometry 4-7.5 50.1 ± 0.5 mV Yes [107]
-1
PANI OCP 4-8 -33.82 mV·pH Yes [108]
Drugs Psychoactive drugs Apt1, Apt2 aptamers Potentiometry 0.001 to 5 μM / Yes [108]
Caffeine Carbon nanotube/Nafion Film DPV 0-40 μM 110 nA·mM -1 Yes [18]
-1
Nicotine CYP2B6 Chronoamperometry 0-30 μM 4.3 nA·mM Yes [97]
-1
Carbamazepine NiSe 2 Chronoamperometry 50 nM to 10 μM 65.65 μA·mM No [109]
rGO: Reduced graphene oxide; PU: polyurethane; PANI: polyaniline; OCP: open-circuit potential; DPV: differential pulse voltammetry; CYP2B6:
cytochrome P450 2B6.
sensors featuring a PVA hydrogel as the sweat collection component. These sensors employ Prussian Blue
(PB) as a transducer to identify the hydrogen peroxide generated through the enzyme-catalyzed glucose
reaction. With the integration of an individualized sweat-to-blood conversion algorithm, these sensors
enable fast, dependable, and user-friendly self-assessment of glucose levels in fingertip sweat [Figure 6A] .
[54]
A textile-based sweat glucose sensor is presented by Toi et al., which utilizes a unique wrinkled, stretchable
nanohybrid fiber (WSNF) as the electrode. This WSNF comprises a hybrid fiber constructed of reduced
graphene oxide (rGO) and polyurethane, with Au nanowrinkles partially covering its surface. Because of the
synergistic impacts of these nanowrinkles and the oxygen-containing functional groups in the rGO-
supporting matrix, the WSNF demonstrates remarkable electrocatalytic activity. These effects significantly
augment the dehydrogenation stage during glucose oxidation. Consequently, the WSNF electrode
demonstrates a remarkable response to glucose, featuring a sensitivity of 140 μA·mM ·cm and a detection
-2
-1
[104]
limit of 500 nM [Figure 6B] .
The ability to detect fluctuations in lactate levels in real-time can indicate physical stress and may aid in
identifying shifts from aerobic to anaerobic states when there is an oxygen shortage and need for extra
energy, as happens during exercise. Figure 6C showcases a lactate sensor patch created through screen
printing on a flexible polyester sheet, designed to perfectly adapt to the intricate contours of the skin,
guaranteeing a distinct signal. The lactate measurement part of the patch features a modified electrode
coated with a bio-compatible layer [lactate oxidase (LOx)-modified PB], with a sensitivity of 96 nA·mM -1[81] .
Saha et al. introduced a seamless platform for continuously monitoring sweat lactate, which integrates an
osmotic hydrogel for sweat collection, a microfluidic channel made of paper for sweat transportation, a
screen-printed electrochemical sensor for lactate detection, and a tailor-made wireless wearable potentiostat
system. At rest, the osmosis hydrogel enables the collection of sweat samples without external power.
Simultaneously, the continuous evaporation in the paper channel allows for prolonged sensing using fresh
sweat. Through strategic placement, lactate sensors offer nearly instant sensing capabilities using minimal