Page 132 - Read Online
P. 132
Page 14 of 19 Kim et al. Soft Sci 2023;3:18 https://dx.doi.org/10.20517/ss.2023.08
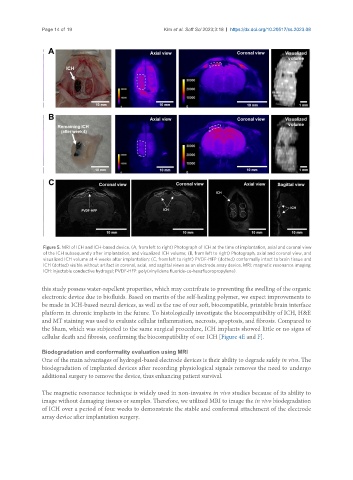
Figure 5. MRI of ICH and ICH-based device. (A, from left to right) Photograph of ICH at the time of implantation, axial and coronal view
of the ICH subsequently after implantation, and visualized ICH volume; (B, from left to right) Photograph, axial and coronal view, and
visualized ICH volume at 4 weeks after implantation; (C, from left to right) PVDF-HFP (dotted) conformally intact to brain tissue and
ICH (dotted) visible without artifact in coronal, axial, and sagittal views as an electrode array device. MRI: magnetic resonance imaging;
ICH: injectable conductive hydrogel; PVDF-HFP: poly(vinylidene fluoride-co-hexafluoropropylene).
this study possess water-repellent properties, which may contribute to preventing the swelling of the organic
electronic device due to biofluids. Based on merits of the self-healing polymer, we expect improvements to
be made in ICH-based neural devices, as well as the use of our soft, biocompatible, printable brain interface
platform in chronic implants in the future. To histologically investigate the biocompatibility of ICH, H&E
and MT staining was used to evaluate cellular inflammation, necrosis, apoptosis, and fibrosis. Compared to
the Sham, which was subjected to the same surgical procedure, ICH implants showed little or no signs of
cellular death and fibrosis, confirming the biocompatibility of our ICH [Figure 4E and F].
Biodegradation and conformality evaluation using MRI
One of the main advantages of hydrogel-based electrode devices is their ability to degrade safely in vivo. The
biodegradation of implanted devices after recording physiological signals removes the need to undergo
additional surgery to remove the device, thus enhancing patient survival.
The magnetic resonance technique is widely used in non-invasive in vivo studies because of its ability to
image without damaging tissues or samples. Therefore, we utilized MRI to image the in vivo biodegradation
of ICH over a period of four weeks to demonstrate the stable and conformal attachment of the electrode
array device after implantation surgery.