Page 131 - Read Online
P. 131
Kim et al. Soft Sci 2023;3:18 https://dx.doi.org/10.20517/ss.2023.08 Page 13 of 19
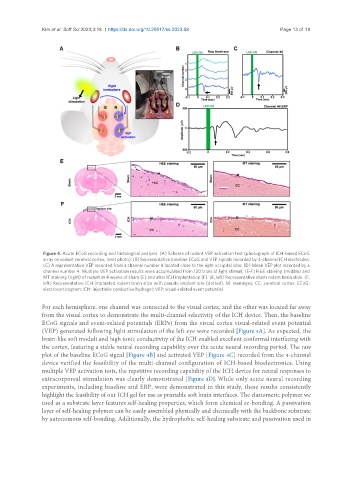
Figure 4. Acute ECoG recording and histological analysis. (A) Scheme of rodent VEP activation test (photograph of ICH-based ECoG
array on rodent cerebral cortex, inset photo); (B) Representative baseline ECoG and VEP signals recorded by 4-channel ICH electrodes;
(C) A representative VEP recorded from a channel number 4 located close to the right occipital lobe; (D) Mean VEP plot recorded by a
channel number 4. Multiple VEP activation results were accumulated from 120 trials of light stimuli; (E-F) H&E staining (middle) and
MT staining (right) of rodent at 4 weeks of sham (E) and after ICH implantation (F). (E, left) Representative sham rodent brain slide. (F,
left) Representative ICH-implanted rodent brain slice with pseudo implant site (dotted). M: meninges; CC: cerebral cortex. ECoG:
electrocorticogram; ICH: injectable conductive hydrogel; VEP: visual-related event potential.
For each hemisphere, one channel was connected to the visual cortex, and the other was located far away
from the visual cortex to demonstrate the multi-channel selectivity of the ICH device. Then, the baseline
ECoG signals and event-related potentials (ERPs) from the visual cortex visual-related event potential
(VEP) generated following light stimulation of the left eye were recorded [Figure 4A]. As expected, the
brain-like soft moduli and high ionic conductivity of the ICH enabled excellent conformal interfacing with
the cortex, featuring a stable neural recording capability over the acute neural recording period. The raw
plot of the baseline ECoG signal [Figure 4B] and activated VEP [Figure 4C] recorded from the 4-channel
device verified the feasibility of the multi-channel configuration of ICH-based bioelectronics. Using
multiple VEP activation tests, the repetitive recording capability of the ICH device for neural responses to
extracorporeal stimulation was clearly demonstrated [Figure 4D]. While only acute neural recording
experiments, including baseline and ERP, were demonstrated in this study, these results consistently
highlight the feasibility of our ICH gel for use as printable soft brain interfaces. The elastomeric polymer we
used as a substrate layer features self-healing properties, which form chemical re-bonding. A passivation
layer of self-healing polymer can be easily assembled physically and chemically with the backbone substrate
by autonomous self-bonding. Additionally, the hydrophobic self-healing substrate and passivation used in