Page 127 - Read Online
P. 127
Kim et al. Soft Sci 2023;3:18 https://dx.doi.org/10.20517/ss.2023.08 Page 9 of 19
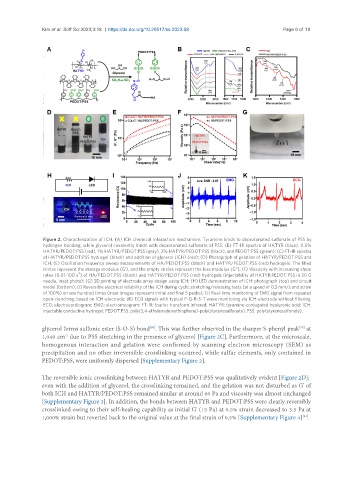
Figure 2. Characterization of ICH. (A) ICH chemical interaction mechanism. Tyramine binds to deprotonated sulfonate of PSS by
hydrogen bonding, while glycerol covalently binds with deprotonated sulfonate of PSS; (B) FT-IR spectra of HATYR (blue), 0.5%
HATYR/PEDOT:PSS (red), 1% HATYR/PEDOT:PSS (gray), 2% HATYR/PEDOT:PSS (black), and PEDOT:PSS (green); (C) FT-IR spectra
of HATYR/PEDOT:PSS hydrogel (black) and addition of glycerol (ICH) (red); (D) Photograph of gelation of HATYR/PEDOT:PSS and
ICH; (E) Oscillation frequency sweep measurements of HA/PEDOT:PSS (black) and HATYR/PEDOT:PSS (red) hydrogels. The filled
circles represent the storage modulus (G'), and the empty circles represent the loss modulus (G"); (F) Viscosity with increasing shear
-1
rates (0.01-100 s ) of HA/PEDOT:PSS (black) and HATYR/PEDOT:PSS (red) hydrogels (injectability of HATYR:PEDOT:PSS in 30 G
needle, inset photo); (G) 3D printing of electrode array design using ICH; (H) LED demonstration of ICH: photograph (top) and circuit
model (bottom); (I) Reversible electrical reliability of the ICH during cyclic stretching/releasing tests (at a speed of 0.3 mm/s and strain
of 100%) on one hundred times (inset images represent initial and final 5 peaks); (J) Real-time monitoring of EMG signal from repeated
open-clenching, based on ICH-electrode; (K) ECG signals with typical P-Q-R-S-T wave monitoring via ICH-electrode without filtering.
ECG: electrocardiogram; EMG: electromyogram; FT-IR: fourier transform infrared; HATYR: tyramine-conjugated hyaluronic acid; ICH:
injectable conductive hydrogel; PEDOT:PSS: poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate); PSS: poly(styrenesulfonate).
[78]
[80]
glycerol forms sulfonic ester (S-O-S) bond . This was further observed in the sharper S-phenyl peak at
1,040 cm due to PSS stretching in the presence of glycerol [Figure 2C]. Furthermore, at the microscale,
-1
homogenous interaction and gelation were confirmed by scanning electron microscopy (SEM) as
precipitation and no other irreversible crosslinking occurred, while sulfur elements, only contained in
PEDOT:PSS, were uniformly dispersed [Supplementary Figure 2].
The reversible ionic crosslinking between HATYR and PEDOT:PSS was qualitatively evident [Figure 2D];
even with the addition of glycerol, the crosslinking remained, and the gelation was not disturbed as G' of
both ICH and HATYR/PEDOT:PSS remained similar at around 60 Pa and viscosity was almost unchanged
[Supplementary Figure 3]. In addition, the bonds between HATYR and PEDOT:PSS were clearly reversibly
crosslinked owing to their self-healing capability as initial G' (15 Pa) at 0.5% strain decreased to 3.5 Pa at
[81]
1,000% strain but reverted back to the original value at the final strain of 0.5% [Supplementary Figure 4] .