Page 59 - Read Online
P. 59
Page 354 vonderEmbse et al. Neuroimmunol Neuroinflammation 2020;7:345-59 I http://dx.doi.org/10.20517/2347-8659.2019.29
A B
C D
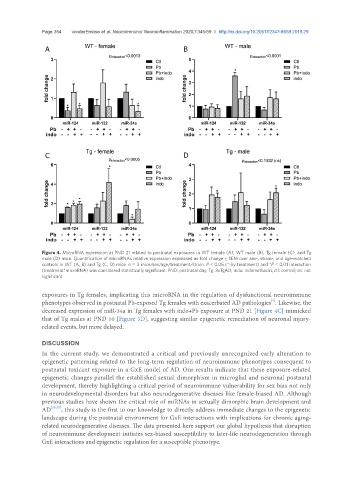
Figure 4. MicroRNA expression at PND 21 related to postnatal exposures in WT female (A), WT male (B), Tg female (C), and Tg
male (D) mice. Quantification of microRNAs relative expression expressed as fold change ± SEM over sex-, strain-, and age-matched
controls in WT (A, B) and Tg (C, D) mice. n = 3 mice/sex/age/treatment/strain. P < 0.05 (*-by treatment) and *P < 0.01 interaction
(treatment*microRNA) was considered statistically significant. PND: postnatal day; Tg: 3xTgAD; indo: indomethacin; ctl: control; ns: not
significant
exposures in Tg females, implicating this microRNA in the regulation of dysfunctional neuroimmune
[4]
phenotypes observed in postnatal Pb-exposed Tg females with exacerbated AD pathologies . Likewise, the
decreased expression of miR-34a in Tg females with indo+Pb exposure at PND 21 [Figure 4C] mimicked
that of Tg males at PND 10 [Figure 3D], suggesting similar epigenetic remediation of neuronal injury-
related events, but more delayed.
DISCUSSION
In the current study, we demonstrated a critical and previously unrecognized early alteration to
epigenetic patterning related to the long-term regulation of neuroimmune phenotypes consequent to
postnatal toxicant exposure in a GxE model of AD. Our results indicate that these exposure-related
epigenetic changes parallel the established sexual dimorphism in microglial and neuronal postnatal
development, thereby highlighting a critical period of neuroimmune vulnerability for sex bias not only
in neurodevelopmental disorders but also neurodegenerative diseases like female-biased AD. Although
previous studies have shown the critical role of miRNAs in sexually dimorphic brain development and
AD [25,32] , this study is the first to our knowledge to directly address immediate changes to the epigenetic
landscape during the postnatal environment for GxE interactions with implications for chronic aging-
related neurodegenerative diseases. The data presented here support our global hypothesis that disruption
of neuroimmune development initiates sex-biased susceptibility to later-life neurodegeneration through
GxE interactions and epigenetic regulation for a susceptible phenotype.