Page 98 - Read Online
P. 98
Ackerman et al. Mini-invasive Surg 2021;5:14 https://dx.doi.org/10.20517/2574-1225.2021.02 Page 17 of 19
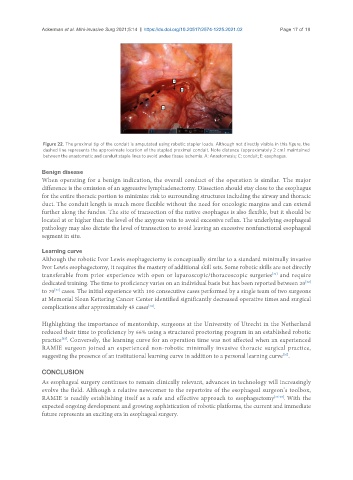
Figure 22. The proximal tip of the conduit is amputated using robotic stapler loads. Although not directly visible in this figure, the
dashed line represents the approximate location of the stapled proximal conduit. Note distance (approximately 2 cm) maintained
between the anastomotic and conduit staple lines to avoid undue tissue ischemia. A: Anastomosis; C: conduit; E: esophagus.
Benign disease
When operating for a benign indication, the overall conduct of the operation is similar. The major
difference is the omission of an aggressive lymphadenectomy. Dissection should stay close to the esophagus
for the entire thoracic portion to minimize risk to surrounding structures including the airway and thoracic
duct. The conduit length is much more flexible without the need for oncologic margins and can extend
further along the fundus. The site of transection of the native esophagus is also flexible, but it should be
located at or higher than the level of the azygous vein to avoid excessive reflux. The underlying esophageal
pathology may also dictate the level of transection to avoid leaving an excessive nonfunctional esophageal
segment in situ.
Learning curve
Although the robotic Ivor Lewis esophagectomy is conceptually similar to a standard minimally invasive
Ivor Lewis esophagectomy, it requires the mastery of additional skill sets. Some robotic skills are not directly
[31]
transferable from prior experience with open or laparoscopic/thoracoscopic surgeries and require
dedicated training. The time to proficiency varies on an individual basis but has been reported between 20
[32]
to 70 cases. The initial experience with 100 consecutive cases performed by a single team of two surgeons
[33]
at Memorial Sloan Kettering Cancer Center identified significantly decreased operative times and surgical
complications after approximately 45 cases .
[34]
Highlighting the importance of mentorship, surgeons at the University of Utrecht in the Netherland
reduced their time to proficiency by 66% using a structured proctoring program in an established robotic
[33]
practice . Conversely, the learning curve for an operation time was not affected when an experienced
RAMIE surgeon joined an experienced non-robotic minimally invasive thoracic surgical practice,
[35]
suggesting the presence of an institutional learning curve in addition to a personal learning curve .
CONCLUSION
As esophageal surgery continues to remain clinically relevant, advances in technology will increasingly
evolve the field. Although a relative newcomer to the repertoire of the esophageal surgeon’s toolbox,
RAMIE is readily establishing itself as a safe and effective approach to esophagectomy [36-38] . With the
expected ongoing development and growing sophistication of robotic platforms, the current and immediate
future represents an exciting era in esophageal surgery.