Page 151 - Read Online
P. 151
Gropman et al. J Transl Genet Genom 2020;4:429-45 I http://dx.doi.org/10.20517/jtgg.2020.09 Page 441
1
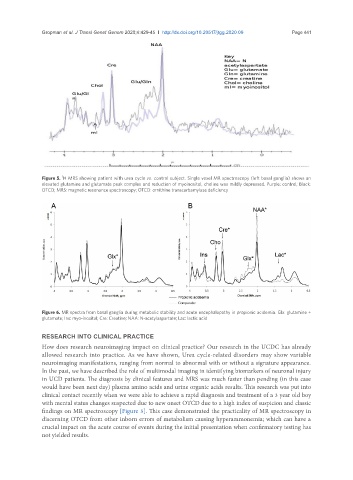
Figure 5. H MRS showing patient with urea cycle vs. control subject. Single voxel MR spectroscopy (left basal ganglia) shows an
elevated glutamine and glutamate peak complex and reduction of myoinositol, choline was mildly depressed. Purple: control; Black:
OTCD; MRS: magnetic resonance spectroscopy; OTCD: ornithine transcarbamylase deficiency
A B
Figure 6. MR spectra from basal ganglia during metabolic stability and acute encephalopathy in propionic acidemia. Glx: glutamine +
glutamate; Ins: myo-inositol; Cre: Creatine; NAA: N-acetylaspartate; Lac: lactic acid
RESEARCH INTO CLINICAL PRACTICE
How does research neuroimaging impact on clinical practice? Our research in the UCDC has already
allowed research into practice. As we have shown, Urea cycle-related disorders may show variable
neuroimaging manifestations, ranging from normal to abnormal with or without a signature appearance.
In the past, we have described the role of multimodal imaging in identifying biomarkers of neuronal injury
in UCD patients. The diagnosis by clinical features and MRS was much faster than pending (in this case
would have been next day) plasma amino acids and urine organic acids results. This research was put into
clinical contact recently when we were able to achieve a rapid diagnosis and treatment of a 3 year old boy
with mental status changes suspected due to new onset OTCD due to a high index of suspicion and classic
findings on MR spectroscopy [Figure 5]. This case demonstrated the practicality of MR spectroscopy in
discerning OTCD from other inborn errors of metabolism causing hyperammonemia; which can have a
crucial impact on the acute course of events during the initial presentation when confirmatory testing has
not yielded results.