Page 126 - Read Online
P. 126
Page 12 of 17 Liu et al. J Mater Inf 2023;3:17 https://dx.doi.org/10.20517/jmi.2023.19
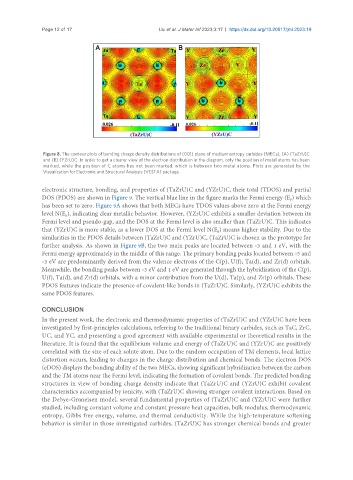
Figure 8. The contour plots of bonding charge density distributions of (001) plane of medium entropy carbides (MECs), (A) (TaZrU)C
and (B) (YZrU)C. In order to get a clearer view of the electron distribution in the diagram, only the position of metal atoms has been
marked, while the position of C atoms has not been marked, which is between two metal atoms. Plots are generated by the
Visualization for Electronic and Structural Analysis (VESTA) package.
electronic structure, bonding, and properties of (TaZrU)C and (YZrU)C, their total (TDOS) and partial
DOS (PDOS) are shown in Figure 9. The vertical blue line in the figure marks the Fermi energy (E ) which
F
has been set to zero. Figure 9A shows that both MECs have TDOS values above zero at the Fermi energy
level N(E ), indicating clear metallic behavior. However, (YZrU)C exhibits a smaller deviation between its
F
Fermi level and pseudo-gap, and the DOS at the Fermi level is also smaller than (TaZrU)C. This indicates
that (YZrU)C is more stable, as a lower DOS at the Fermi level N(E ) means higher stability. Due to the
F
similarities in the PDOS details between (TaZrU)C and (YZrU)C, (TaZrU)C is chosen as the prototype for
further analysis. As shown in Figure 9B, the two main peaks are located between -3 and 1 eV, with the
Fermi energy approximately in the middle of this range. The primary bonding peaks located between -5 and
-3 eV are predominantly derived from the valence electrons of the C(p), U(f), Ta(d), and Zr(d) orbitals.
Meanwhile, the bonding peaks between -3 eV and 1 eV are generated through the hybridization of the C(p),
U(f), Ta(d), and Zr(d) orbitals, with a minor contribution from the U(d), Ta(p), and Zr(p) orbitals. These
PDOS features indicate the presence of covalent-like bonds in (TaZrU)C. Similarly, (YZrU)C exhibits the
same PDOS features.
CONCLUSION
In the present work, the electronic and thermodynamic properties of (TaZrU)C and (YZrU)C have been
investigated by first-principles calculations, referring to the traditional binary carbides, such as TaC, ZrC,
UC, and YC, and presenting a good agreement with available experimental or theoretical results in the
literature. It is found that the equilibrium volume and energy of (TaZrU)C and (YZrU)C are positively
correlated with the size of each solute atom. Due to the random occupation of TM elements, local lattice
distortion occurs, leading to changes in the charge distribution and chemical bonds. The electron DOS
(eDOS) displays the bonding ability of the two MECs, showing significant hybridization between the carbon
and the TM atoms near the Fermi level, indicating the formation of covalent bonds. The predicted bonding
structures in view of bonding charge density indicate that (TaZrU)C and (YZrU)C exhibit covalent
characteristics accompanied by ionicity, with (TaZrU)C showing stronger covalent interactions. Based on
the Debye-Grüneisen model, several fundamental properties of (TaZrU)C and (YZrU)C were further
studied, including constant volume and constant pressure heat capacities, bulk modulus, thermodynamic
entropy, Gibbs free energy, volume, and thermal conductivity. While the high-temperature softening
behavior is similar in those investigated carbides, (TaZrU)C has stronger chemical bonds and greater