Page 122 - Read Online
P. 122
Page 8 of 17 Liu et al. J Mater Inf 2023;3:17 https://dx.doi.org/10.20517/jmi.2023.19
Table 2. Based on quasiharmonic approximation, thermodynamic properties of (TaZrU)C and (YZrU)C predicted by first-principles
calculations, including equilibrium volume (V ), Internal energy, Gibbs free energy, Constant volume heat capacity (Cv), Constant
0
pressure heat capacity (Cp), and Isothermal bulk modulus (B )
0
V Internal Gibbs Cv Cp B
Composition 0 3 0
(Å /atom) (eV) (eV) (J/mol/K) (J/mol/K) (GPa)
(TaZrU)C 13.446 -9.6614 -9.7428 23.098 23.412 228.34
(YZrU)C 15.211 -8.8405 -8.9249 23.174 23.610 167.46
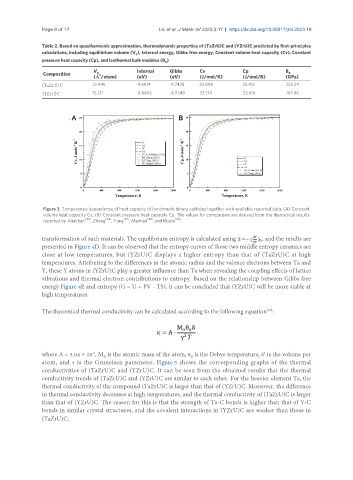
Figure 3. Temperature dependence of heat capacity of benchmark binary carbides together with available reported data. (A) Constant
volume heat capacity Cv; (B) Constant pressure heat capacity Cp. The values for comparison are derived from the theoretical results
[93] [94] [57] [95] [96]
reported by Aliakbari , Zhang , Yang , Mankad , and Iikubo .
transformation of such materials. The equilibrium entropy is calculated using S = -(∂F) , and the results are
V
presented in Figure 4D. It can be observed that the entropy curves of those two middle entropy ceramics are
close at low temperatures, but (YZrU)C displays a higher entropy than that of (TaZrU)C at high
temperatures. Attributing to the differences in the atomic radius and the valence electrons between Ta and
Y, these Y atoms in (YZrU)C play a greater influence than Ta when revealing the coupling effects of lattice
vibrations and thermal electron contributions to entropy. Based on the relationship between Gibbs free
energy Figure 4E and entropy (G = U + PV - TS), it can be concluded that (YZrU)C will be more stable at
high temperatures.
The theoretical thermal conductivity can be calculated according to the following equation :
[92]
-8
where A = 3.04 × 10 , M is the atomic mass of the atom, θ is the Debye temperature, δ is the volume per
3
α
α
atom, and γ is the Gruneisen parameter. Figure 5 shows the corresponding graphs of the thermal
conductivities of (TaZrU)C and (YZrU)C. It can be seen from the obtained results that the thermal
conductivity trends of (TaZrU)C and (YZrU)C are similar to each other. For the heavier element Ta, the
thermal conductivity of the compound (TaZrU)C is larger than that of (YZrU)C. Moreover, the difference
in thermal conductivity decreases at high temperatures, and the thermal conductivity of (TaZrU)C is larger
than that of (YZrU)C. The reason for this is that the strength of Ta-C bonds is higher than that of Y-C
bonds in similar crystal structures, and the covalent interactions in (YZrU)C are weaker than those in
(TaZrU)C.