Page 125 - Read Online
P. 125
Liu et al. J Mater Inf 2023;3:17 https://dx.doi.org/10.20517/jmi.2023.19 Page 11 of 17
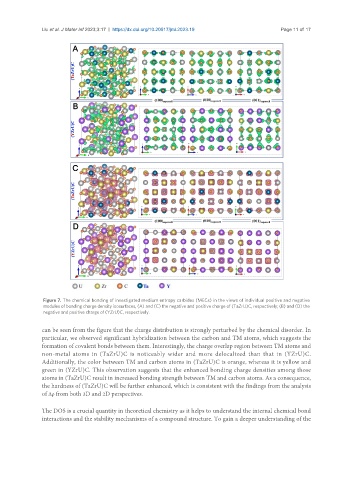
Figure 7. The chemical bonding of investigated medium entropy carbides (MECs) in the views of individual positive and negative
modules of bonding charge density isosurfaces, (A) and (C) the negative and positive charge of (TaZrU)C, respectively; (B) and (D) the
negative and positive charge of (YZrU)C, respectively.
can be seen from the figure that the charge distribution is strongly perturbed by the chemical disorder. In
particular, we observed significant hybridization between the carbon and TM atoms, which suggests the
formation of covalent bonds between them. Interestingly, the charge overlap region between TM atoms and
non-metal atoms in (TaZrU)C is noticeably wider and more delocalized than that in (YZrU)C.
Additionally, the color between TM and carbon atoms in (TaZrU)C is orange, whereas it is yellow and
green in (YZrU)C. This observation suggests that the enhanced bonding charge densities among those
atoms in (TaZrU)C result in increased bonding strength between TM and carbon atoms. As a consequence,
the hardness of (TaZrU)C will be further enhanced, which is consistent with the findings from the analysis
of Δρ from both 3D and 2D perspectives.
The DOS is a crucial quantity in theoretical chemistry as it helps to understand the internal chemical bond
interactions and the stability mechanisms of a compound structure. To gain a deeper understanding of the