Page 161 - Read Online
P. 161
Tian et al. EMT drives anti-estrogen resistance in breast cancer
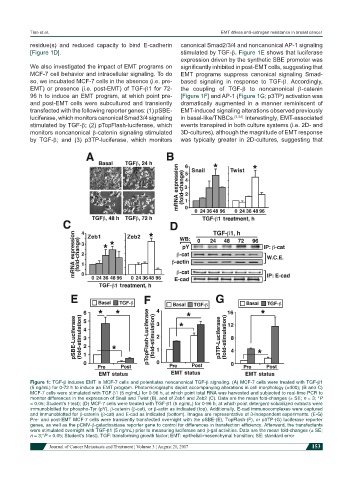
residue(s) and reduced capacity to bind E-cadherin canonical Smad2/3/4 and noncanonical AP-1 signaling
[Figure 1D]. stimulated by TGF-β. Figure 1E shows that luciferase
expression driven by the synthetic SBE promoter was
We also investigated the impact of EMT programs on significantly inhibited in post-EMT cells, suggesting that
MCF-7 cell behavior and intracellular signaling. To do EMT programs suppress canonical signaling Smad-
so, we incubated MCF-7 cells in the absence (i.e. pre- based signaling in response to TGF-β. Accordingly,
EMT) or presence (i.e. post-EMT) of TGF-β1 for 72- the coupling of TGF-β to noncanonical β-catenin
96 h to induce an EMT program, at which point pre- [Figure 1F] and AP-1 (Figure 1G; p3TP) activation was
and post-EMT cells were subcultured and transiently dramatically augmented in a manner reminiscent of
transfected with the following reporter genes: (1) pSBE- EMT-induced signaling alterations observed previously
luciferase, which monitors canonical Smad3/4 signaling in basal-like/TNBCs. [4,24] Interestingly, EMT-associated
stimulated by TGF-β; (2) pTopFlash-luciferase, which events transpired in both culture systems (i.e. 2D- and
monitors noncanonical β-catenin signaling stimulated 3D-cultures), although the magnitude of EMT response
by TGF-β; and (3) p3TP-luciferase, which monitors was typically greater in 2D-cultures, suggesting that
Figure 1: TGF-β induces EMT in MCF-7 cells and potentiates noncanonical TGF-β signaling. (A) MCF-7 cells were treated with TGF-β1
(5 ng/mL) for 0-72 h to induce an EMT program. Photomicrographs depict accompanying alterations in cell morphology (×400); (B and C)
MCF-7 cells were stimulated with TGF-β1 (5 ng/mL) for 0-96 h, at which point total RNA was harvested and subjected to real-time PCR to
monitor differences in the expression of Snail and Twist (B), and of Zeb1 and Zeb2 (C). Data are the mean fold-changes (± SE; n = 3; *P
< 0.05; Student’s t-test); (D) MCF-7 cells were treated with TGF-β1 (5 ng/mL) for 0-96 h, at which point detergent-solubilized extracts were
immunoblotted for phospho-Tyr (pY), β-catenin (β-cat), or β-actin as indicated (top). Additionally, E-cad immunocomplexes were captured
and immunoblotted for β-catenin (β-cat) and E-cad as indicated (bottom). Images are representative of 3-independent experiments. (E-G)
Pre- and post-EMT MCF-7 cells were transiently transfected overnight with the pSBE-(E), TopFlash-(F), or p3TP-(G) luciferase reporter
genes, as well as the pCMV-β-galactosidase reporter gene to control for differences in transfection efficiency. Afterward, the transfectants
were stimulated overnight with TGF-β1 (5 ng/mL) prior to measuring luciferase and β-gal activities. Data are the mean fold-changes (± SE;
n = 3;*P < 0.05; Student’s t-test). TGF: transforming growth factor; EMT: epithelial-mesenchymal transition; SE: standard error
Journal of Cancer Metastasis and Treatment ¦ Volume 3 ¦ August 21, 2017 153