Page 47 - Read Online
P. 47
Saier et al. J Cancer Metastasis Treat 2021;7:43 https://dx.doi.org/10.20517/2394-4722.2021.87 Page 9 of 24
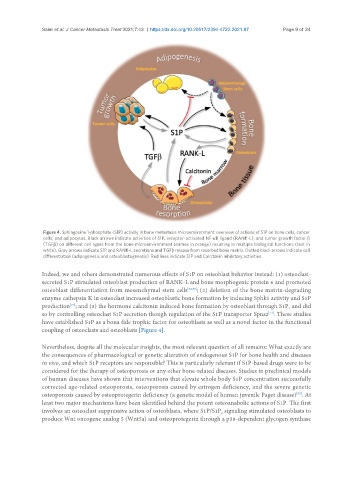
Figure 4. Sphingosine 1-phosphate (S1P) activity in bone metastasis microenvironment: overview of actions of S1P on bone cells, cancer
cells, and adipocytes. Black arrows indicate activities of S1P, receptor-activated NF-κB ligand (RANK-L), and tumor growth factor β
(TGFβ) on different cell types from the bone microenvironment (names in orange) resulting in multiple biological functions (text in
white). Grey arrows indicate S1P and RANK-L secretions and TGFβ release from resorbed bone matrix. Dotted black arrows indicate cell
differentiation (adipogenesis and osteoblastogenesis). Red lines indicate S1P and Calcitonin inhibitory activities.
Indeed, we and others demonstrated numerous effects of S1P on osteoblast behavior instead: (1) osteoclast-
secreted S1P stimulated osteoblast production of RANK-L and bone morphogenic protein 6 and promoted
osteoblast differentiation from mesenchymal stem cells [72,76] ; (2) deletion of the bone matrix-degrading
enzyme cathepsin K in osteoclast increased osteoblastic bone formation by inducing Sphk1 activity and S1P
production ; and (3) the hormone calcitonin induced bone formation by osteoblast through S1P and did
[77]
3
so by controlling osteoclast S1P secretion though regulation of the S1P transporter Spns2 . These studies
[71]
have established S1P as a bona fide trophic factor for osteoblasts as well as a novel factor in the functional
coupling of osteoclasts and osteoblasts [Figure 4].
Nevertheless, despite all the molecular insights, the most relevant question of all remains: What exactly are
the consequences of pharmacological or genetic alteration of endogenous S1P for bone health and diseases
in vivo, and which S1P receptors are responsible? This is particularly relevant if S1P-based drugs were to be
considered for the therapy of osteoporosis or any other bone-related diseases. Studies in preclinical models
of human diseases have shown that interventions that elevate whole body S1P concentration successfully
corrected age-related osteoporosis, osteoporosis caused by estrogen deficiency, and the severe genetic
osteoporosis caused by osteoprotegerin deficiency (a genetic model of human juvenile Paget disease) . At
[75]
least two major mechanisms have been identified behind the potent osteoanabolic actions of S1P. The first
involves an osteoclast suppressive action of osteoblasts, where S1P/S1P signaling stimulated osteoblasts to
2
produce Wnt oncogene analog 5 (Wnt5a) and osteoprotegerin through a p38-dependent glycogen synthase