Page 46 - Read Online
P. 46
Page 8 of 24 Saier et al. J Cancer Metastasis Treat 2021;7:43 https://dx.doi.org/10.20517/2394-4722.2021.87
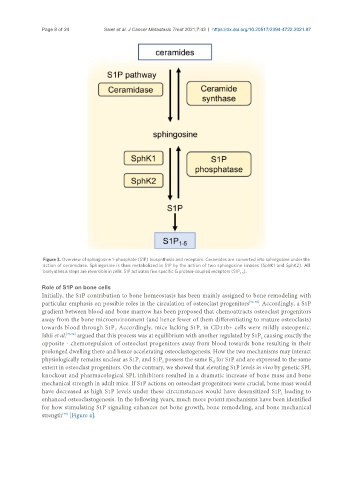
Figure 3. Overview of sphingosine 1-phosphate (S1P) biosynthesis and receptors. Ceramides are converted into sphingosine under the
action of ceramidase. Sphingosine is then metabolized in S1P by the action of two sphingosine kinases (SphK1 and SphK2). All
biosynthesis steps are reversible in cells. S1P activates five specific G protein-coupled receptors (S1P ).
1-5
Role of S1P on bone cells
Initially, the S1P contribution to bone homeostasis has been mainly assigned to bone remodeling with
particular emphasis on possible roles in the circulation of osteoclast progenitors [70-72] . Accordingly, a S1P
gradient between blood and bone marrow has been proposed that chemoattracts osteoclast progenitors
away from the bone microenvironment (and hence fewer of them differentiating to mature osteoclasts)
towards blood through S1P . Accordingly, mice lacking S1P in CD11b+ cells were mildly osteopenic.
1
1
Ishii et al. [73,74] argued that this process was at equilibrium with another regulated by S1P causing exactly the
2
opposite - chemorepulsion of osteoclast progenitors away from blood towards bone resulting in their
prolonged dwelling there and hence accelerating osteoclastogenesis. How the two mechanisms may interact
physiologically remains unclear as S1P and S1P possess the same K for S1P and are expressed to the same
1
d
2
extent in osteoclast progenitors. On the contrary, we showed that elevating S1P levels in vivo by genetic SPL
knockout and pharmacological SPL inhibitors resulted in a dramatic increase of bone mass and bone
mechanical strength in adult mice. If S1P actions on osteoclast progenitors were crucial, bone mass would
have decreased as high S1P levels under these circumstances would have desensitized S1P leading to
1
enhanced osteoclastogenesis. In the following years, much more potent mechanisms have been identified
for how stimulating S1P signaling enhances net bone growth, bone remodeling, and bone mechanical
strength [Figure 4].
[75]