Page 45 - Read Online
P. 45
Tu et al. J Cancer Metastasis Treat 2018;4:58 I http://dx.doi.org/10.20517/2394-4722.2018.67 Page 11 of 16
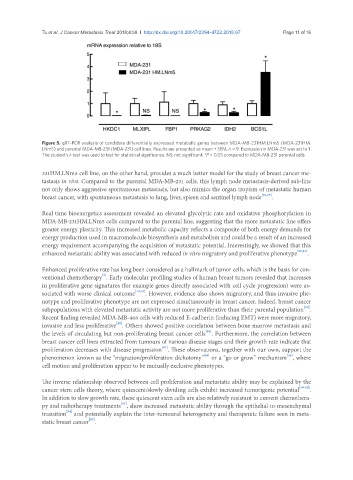
Figure 5. qRT-PCR analysis of candidate differentially expressed metabolic genes between MDA-MB-231HM.LNm5 (MDA-231HM.
LNm5) and parental MDA-MB-231 (MDA-231) cell lines. Results are presented as mean ± SEM, n = 9. Expression in MDA-231 was set to 1.
The student’s t-test was used to test for statistical significance. NS: not significant; *P < 0.05 compared to MDA-MB-231 parental cells
231HM.LNm5 cell line, on the other hand, provides a much better model for the study of breast cancer me-
tastasis in vivo. Compared to the parental MDA-MB-231 cells, this lymph node metastasis-derived sub-line
not only shows aggressive spontaneous metastasis, but also mimics the organ tropism of metastatic human
breast cancer, with spontaneous metastasis to lung, liver, spleen and sentinel lymph node [22,23] .
Real time bioenergetics assessment revealed an elevated glycolytic rate and oxidative phosphorylation in
MDA-MB-231HM.LNm5 cells compared to the parental line, suggesting that the more metastatic line offers
greater energy plasticity. This increased metabolic capacity reflects a composite of both energy demands for
energy production used in macromolecule biosynthesis and metabolism and could be a result of an increased
energy requirement accompanying the acquisition of metastatic potential. Interestingly, we showed that this
enhanced metastatic ability was associated with reduced in vitro migratory and proliferative phenotype [22,23] .
Enhanced proliferative rate has long been considered as a hallmark of tumor cells, which is the basis for con-
[5]
ventional chemotherapy . Early molecular profiling studies of human breast tumors revealed that increases
in proliferative gene signatures (for example genes directly associated with cell cycle progression) were as-
sociated with worse clinical outcome [42,43] . However, evidence also shows migratory, and thus invasive phe-
notype and proliferative phenotype are not expressed simultaneously in breast cancer. Indeed, breast cancer
[44]
subpopulations with elevated metastatic activity are not more proliferative than their parental population .
Recent finding revealed MDA-MB-468 cells with reduced E-cadherin (inducing EMT) were more migratory,
[45]
invasive and less proliferative . Others showed positive correlation between bone marrow metastasis and
[46]
the levels of circulating but non-proliferating breast cancer cells . Furthermore, the correlation between
breast cancer cell lines extracted from tumours of various disease stages and their growth rate indicate that
[47]
proliferation decreases with disease progression . These observations, together with our own, support the
[48]
phenomenon known as the “migration/proliferation dichotomy” or a “go or grow” mechanism , where
[49]
cell motion and proliferation appear to be mutually exclusive phenotypes.
The inverse relationship observed between cell proliferation and metastatic ability may be explained by the
cancer stem cells theory, where quiescent/slowly dividing cells exhibit increased tumorigenic potential [50-52] .
In addition to slow growth rate, these quiescent stem cells are also relatively resistant to current chemothera-
py and radiotherapy treatments , show increased metastatic ability through the epithelial-to-mesenchymal
[53]
[54]
transition and potentially explain the inter-tumoural heterogeneity and therapeutic failure seen in meta-
static breast cancer .
[55]