Page 382 - Read Online
P. 382
Verkoeijen et al. J Cancer Metastasis Treat 2019;5:51 I http://dx.doi.org/10.20517/2394-4722.2019.06 Page 11 of 16
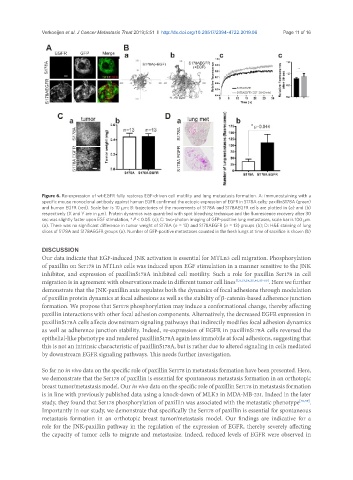
Figure 6. Re-expression of wt-EGFR fully restores EGF-driven cell motility and lung metastasis formation. A: immunostaining with a
specific mouse monoclonal antibody against human EGFR confirmed the ectopic expression of EGFR in S178A cells; paxillinS178A (green)
and human EGFR (red). Scale bar is 10 mm; B: trajectories of the movements of S178A and S178AEGFR cells are plotted in (a) and (b)
respectively (X and Y are in mm). Protein dynamics was quantified with spot bleaching technique and the fluorescence recovery after 30
sec was slightly faster upon EGF stimulation, * P < 0.05. (c); C: two-photon imaging of GFP-positive lung metastases, scale bar is 100 mm.
(a). There was no significant difference in tumor weight of S178A (n = 13) and S178AEGFR (n = 13) groups (b); D: H&E staining of lung
slices of S178A and S178AEGFR groups (a). Number of GFP-positive metastases counted in the fresh lungs at time of sacrifice is shown (b)
DISCUSSION
Our data indicate that EGF-induced JNK activation is essential for MTLn3 cell migration. Phosphorylation
of paxillin on Ser178 in MTLn3 cells was induced upon EGF stimulation in a manner sensitive to the JNK
inhibitor, and expression of paxillinS178A inhibited cell motility. Such a role for paxillin Ser178 in cell
migration is in agreement with observations made in different tumor cell lines [7,9,13,24,25,40,55-60] . Here we further
demonstrate that the JNK-paxillin axis regulates both the dynamics of focal adhesions through modulation
of paxillin protein dynamics at focal adhesions as well as the stability of β-catenin-based adherence junction
formation. We propose that Ser178 phosphorylation may induce a conformational change, thereby affecting
paxillin interactions with other focal adhesion components. Alternatively, the decreased EGFR expression in
paxillinS178A cells affects downstream signaling pathways that indirectly modifies focal adhesion dynamics
as well as adherence junction stability. Indeed, re-expression of EGFR in paxillinS178A cells reversed the
epithelial-like phenotype and rendered paxillinS178A again less immobile at focal adhesions, suggesting that
this is not an intrinsic characteristic of paxillinS178A, but is rather due to altered signaling in cells mediated
by downstream EGFR signaling pathways. This needs further investigation.
So far no in vivo data on the specific role of paxillin Ser178 in metastasis formation have been presented. Here,
we demonstrate that the Ser178 of paxillin is essential for spontaneous metastasis formation in an orthotopic
breast tumor/metastasis model. Our in vivo data on the specific role of paxillin Ser178 in metastasis formation
is in line with previously published data using a knock-down of MLK3 in MDA-MB-231. Indeed in the later
study, they found that Ser178 phosphorylation of paxillin was associated with the metastatic phenotype [40,59] .
Importantly in our study, we demonstrate that specifically the Ser178 of paxillin is essential for spontaneous
metastasis formation in an orthotopic breast tumor/metastasis model. Our findings are indicative for a
role for the JNK-paxillin pathway in the regulation of the expression of EGFR, thereby severely affecting
the capacity of tumor cells to migrate and metastasize. Indeed, reduced levels of EGFR were observed in