Page 377 - Read Online
P. 377
Page 6 of 16 Verkoeijen et al. J Cancer Metastasis Treat 2019;5:51 I http://dx.doi.org/10.20517/2394-4722.2019.06
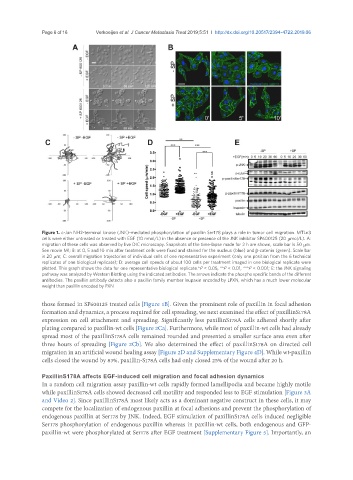
Figure 1. c-Jun NH2-terminal kinase (JNK)-mediated phosphorylation of paxillin Ser178 plays a role in tumor cell migration. MTLn3
cells were either untreated or treated with EGF (10 nmol/L) in the absence or presence of the JNK inhibitor SP600125 (20 mmol/L). A:
migration of these cells was observed by live DIC microscopy. Snapshots of the time-lapse made for 2 h are shown, scale bar is 50 mm.
See movie M1; B: at 0, 5 and 10 min after treatment cells were fixed and stained for the nucleus (blue) and β-catenin (green). Scale bar
is 20 mm; C: overall migration trajectories of individual cells of one representative experiment (only one position from the 6 technical
replicates of one biological replicate); D: average cell speeds of about 100 cells per treatment imaged in one biological replicate were
plotted. This graph shows the data for one representative biological replicate.*P < 0.05, **P < 0.01, ***P < 0.001; E: the JNK signaling
pathway was analyzed by Western Blotting using the indicated antibodies. The arrows indicate the phospho specific bands of the different
antibodies. The paxillin antibody detects also a paxillin family member leupaxin encoded by LPXN, which has a much lower molecular
weight than paxillin encoded by PXN
those formed in SP600125 treated cells [Figure 1B]. Given the prominent role of paxillin in focal adhesion
formation and dynamics, a process required for cell spreading, we next examined the effect of paxillinS178A
expression on cell attachment and spreading. Significantly less paxillinS178A cells adhered shortly after
plating compared to paxillin-wt cells [Figure 2Ca]. Furthermore, while most of paxillin-wt cells had already
spread most of the paxillinS178A cells remained rounded and presented a smaller surface area even after
three hours of spreading [Figure 2Cb]. We also determined the effect of paxillinS178A on directed cell
migration in an artificial wound healing assay [Figure 2D and Supplementary Figure 4D]. While wt-paxillin
cells closed the wound by 83%, paxillin-S178A cells had only closed 25% of the wound after 20 h.
PaxillinS178A affects EGF-induced cell migration and focal adhesion dynamics
In a random cell migration assay paxillin-wt cells rapidly formed lamellipodia and became highly motile
while paxillinS178A cells showed decreased cell motility and responded less to EGF stimulation [Figure 3A
and Video 2]. Since paxillinS178A most likely acts as a dominant negative construct in these cells, it may
compete for the localization of endogenous paxillin at focal adhesions and prevent the phosphorylation of
endogenous paxillin at Ser178 by JNK. Indeed, EGF stimulation of paxillinS178A cells induced negligible
Ser178 phosphorylation of endogenous paxillin whereas in paxillin-wt cells, both endogenous and GFP-
paxillin-wt were phosphorylated at Ser178 after EGF treatment [Supplementary Figure 5]. Importantly, an