Page 17 - Read Online
P. 17
de Santis et al. Hepatoma Res 2019;5:1 I http://dx.doi.org/10.20517/2394-5079.2018.65 Page 9 of 16
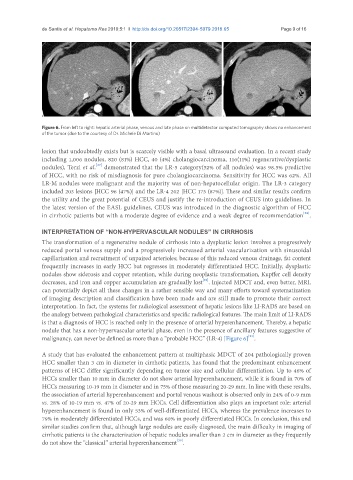
Figure 6. From left to right: hepatic arterial phase, venous and late phase on multidetector computed tomography shows no enhancement
of the tumor (due to the courtesy of Dr. Michele Di Martino)
lesion that undoubtedly exists but is scarcely visible with a basal ultrasound evaluation. In a recent study
including 1,006 nodules, 820 (81%) HCC, 40 (4%) cholangiocarcinoma, 116(11%) regenerative/dysplastic
[27]
nodules), Terzi et al. demonstrated that the LR-5 category(52% of all nodules) was 98.5% predictive
of HCC, with no risk of misdiagnosis for pure cholangiocarcinoma. Sensitivity for HCC was 62%. All
LR-M nodules were malignant and the majority was of non-hepatocellular origin. The LR-3 category
included 203 lesions [HCC 96 (47%)] and the LR-4 202 [HCC 173 (87%)]. These and similar results confirm
the utility and the great potential of CEUS and justify the re-introduction of CEUS into guidelines. In
the latest version of the EASL guidelines, CEUS was introduced in the diagnostic algorithm of HCC
[28]
in cirrhotic patients but with a moderate degree of evidence and a weak degree of recommendation .
INTERPRETATION OF “NON-HYPERVASCULAR NODULES” IN CIRRHOSIS
The transformation of a regenerative nodule of cirrhosis into a dysplastic lesion involves a progressively
reduced portal venous supply and a progressively increased arterial vascularization with sinusoidal
capillarization and recruitment of unpaired arterioles; because of this reduced venous drainage, fat content
frequently increases in early HCC but regresses in moderately differentiated HCC. Initially, dysplastic
nodules show siderosis and copper retention, while during neoplastic transformation, Kupffer cell density
[29]
decreases, and iron and copper accumulation are gradually lost . Injected MDCT and, even better, MRI,
can potentially depict all these changes in a rather sensible way and many efforts toward systematization
of imaging description and classification have been made and are still made to promote their correct
interpretation. In fact, the systems for radiological assessment of hepatic lesions like LI-RADS are based on
the analogy between pathological characteristics and specific radiological features. The main limit of LI-RADS
is that a diagnosis of HCC is reached only in the presence of arterial hyperenhancement. Thereby, a hepatic
nodule that has a non-hypervascular arterial phase, even in the presence of ancillary features suggestive of
[21]
malignancy, can never be defined as more than a “probable HCC” (LR-4) [Figure 6] .
A study that has evaluated the enhancement pattern at multiphasic MDCT of 204 pathologically proven
HCC smaller than 3 cm in diameter in cirrhotic patients, has found that the predominant enhancement
patterns of HCC differ significantly depending on tumor size and cellular differentiation. Up to 46% of
HCCs smaller than 10 mm in diameter do not show arterial hyperenhancement, while it is found in 70% of
HCCs measuring 10-19 mm in diameter and in 75% of those measuring 20-29 mm. In line with these results,
the association of arterial hyperenhancement and portal venous washout is observed only in 24% of 0-9 mm
vs. 28% of 10-19 mm vs. 47% of 20-29 mm HCCs. Cell differentiation also plays an important role: arterial
hyperenhancement is found in only 53% of well-differentiated HCCs, whereas the prevalence increases to
79% in moderately differentiated HCCs, and was 60% in poorly differentiated HCCs. In conclusion, this and
similar studies confirm that, although large nodules are easily diagnosed, the main difficulty in imaging of
cirrhotic patients is the characterization of hepatic nodules smaller than 2 cm in diameter as they frequently
do not show the “classical” arterial hyperenhancement .
[30]