Page 68 - Read Online
P. 68
Liu et al. Chem Synth 2023;3:24 https://dx.doi.org/10.20517/cs.2023.13 Page 9 of 14
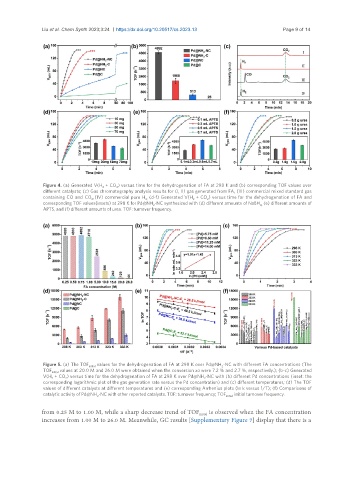
Figure 4. (a) Generated V(H + CO ) versus time for the dehydrogenation of FA at 298 K and (b) corresponding TOF values over
2 2
different catalysts; (c) Gas chromatography analysis results for (I, II) gas generated from FA, (III) commercial mixed standard gas
containing CO and CO , (IV) commercial pure H (d-f) Generated V(H + CO ) versus time for the dehydrogenation of FA and
2 2; 2 2
corresponding TOF values(insets) at 298 K for Pd@NH -NC synthesized with (d) different amounts of NaBH (e) different amounts of
2 4,
APTS, and (f) different amounts of urea. TOF: turnover frequency.
Figure 5. (a) The TOF initial values for the dehydrogenation of FA at 298 K over Pd@NH -NC with different FA concentrations (The
2
TOF initial values at 20.0 M and 26.0 M were obtained when the conversion xa were 7.2 % and 2.7 %, respectively.); (b-c) Generated
V(H + CO ) versus time for the dehydrogenation of FA at 298 K over Pd@NH -NC with (b) different Pd concentrations (inset: the
2
2
2
corresponding logarithmic plot of the gas generation rate versus the Pd concentration) and (c) different temperatures; (d) The TOF
values of different catalysts at different temperatures and (e) corresponding Arrhenius plots (ln k versus 1/T); (f) Comparisons of
catalytic activity of Pd@NH -NC with other reported catalysts. TOF: turnover frequency; TOF initial: initial turnover frequency.
2
from 0.25 M to 1.00 M, while a sharp decrease trend of TOF initial is observed when the FA concentration
increases from 1.00 M to 26.0 M. Meanwhile, GC results [Supplementary Figure 7] display that there is a