Page 68 - Read Online
P. 68
Page 32 of 54 Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38
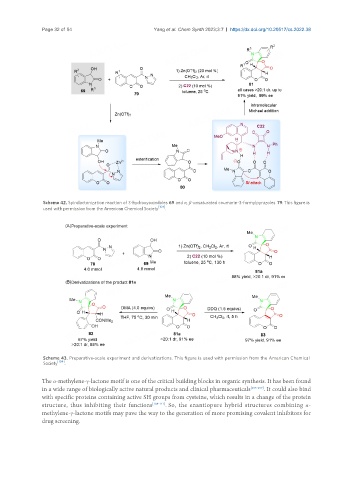
Scheme 42. Spirolactonization reaction of 3-hydroxyoxindoles 69 and α, β-unsaturated coumarin-3-formylpyrazoles 79. This figure is
used with permission from the American Chemical Society [104] .
Scheme 43. Preparative-scale experiment and derivatizations. This figure is used with permission from the American Chemical
Society [104] .
The α-methylene-γ-lactone motif is one of the critical building blocks in organic synthesis. It has been found
in a wide range of biologically active natural products and clinical pharmaceuticals [105-107] . It could also bind
with specific proteins containing active SH groups from cysteine, which results in a change of the protein
structure, thus inhibiting their functions [108-111] . So, the enantiopure hybrid structures combining α-
methylene-γ-lactone motifs may pave the way to the generation of more promising covalent inhibitors for
drug screening.