Page 70 - Read Online
P. 70
Page 34 of 54 Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38
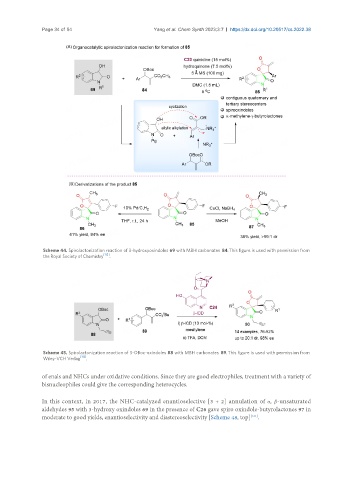
Scheme 44. Spirolactonization reaction of 3-hydroxyoxindoles 69 with MBH carbonates 84. This figure is used with permission from
the Royal Society of Chemistry [112] .
Scheme 45. Spirolactonization reaction of 3-OBoc-oxindoles 88 with MBH carbonates 89. This figure is used with permission from
Wiley-VCH Verlag [113] .
of enals and NHCs under oxidative conditions. Since they are good electrophiles, treatment with a variety of
bisnucleophiles could give the corresponding heterocycles.
In this context, in 2017, the NHC-catalyzed enantioselective [3 + 2] annulation of α, β-unsaturated
aldehydes 95 with 3-hydroxy oxindoles 69 in the presence of C26 gave spiro oxindole-butyrolactones 97 in
[121]
moderate to good yields, enantioselectivity and diastereoselectivity [Scheme 48, top] .