Page 67 - Read Online
P. 67
Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38 Page 31 of 54
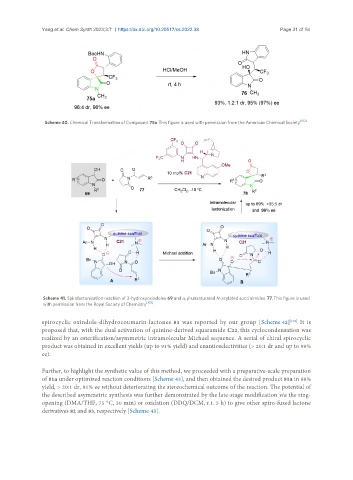
Scheme 40. Chemical Transformation of Compound 75a. This figure is used with permission from the American Chemical Society [102] .
Scheme 41. Spirolactonization reaction of 3-hydroxyoxindoles 69 and α, β-unsaturated N-acylated succinimides 77. This figure is used
with permission from the Royal Society of Chemistry [103] .
spirocyclic oxindole-dihydrocoumarin-lactones 81 was reported by our group [Scheme 42] . It is
[104]
proposed that, with the dual activation of quinine-derived squaramide C22, this cyclocondensation was
realized by an esterification/asymmetric intramolecular Michael sequence. A serial of chiral spirocyclic
product was obtained in excellent yields (up to 91% yield) and enantioselectivities (> 20:1 dr and up to 99%
ee).
Further, to highlight the synthetic value of this method, we proceeded with a preparative-scale preparation
of 81a under optimized reaction conditions [Scheme 43], and then obtained the desired product 81a in 88%
yield, > 20:1 dr, 91% ee without deteriorating the stereochemical outcome of the reaction. The potential of
the described asymmetric synthesis was further demonstrated by the late-stage modification via the ring-
opening (DMA/THF, 75 °C, 30 min) or oxidation (DDQ/DCM, r.t. 5 h) to give other spiro-fused lactone
derivatives 82 and 83, respectively [Scheme 43].