Page 66 - Read Online
P. 66
Page 30 of 54 Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38
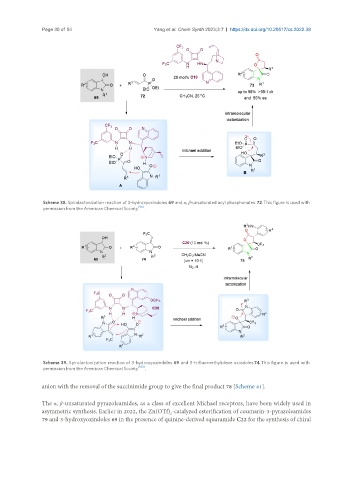
Scheme 38. Spirolactonization reaction of 3-hydroxyoxindoles 69 and α, β-unsaturated acyl phosphonates 72. This figure is used with
permission from the American Chemical Society [96] .
Scheme 39. Spirolactonization reaction of 3-hydroxyoxindoles 69 and 3-trifluoroethylidene oxindoles74. This figure is used with
permission from the American Chemical Society [102] .
anion with the removal of the succinimide group to give the final product 78 [Scheme 41].
The α, β-unsaturated pyrazoleamides, as a class of excellent Michael receptors, have been widely used in
asymmetric synthesis. Earlier in 2022, the Zn(OTf) -catalyzed esterification of coumarin-3-pyrazoleamides
2
79 and 3-hydroxyoxindoles 69 in the presence of quinine-derived squaramide C22 for the synthesis of chiral