Page 75 - Read Online
P. 75
Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38 Page 39 of 54
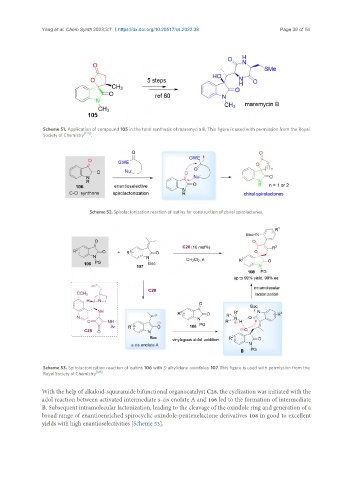
Scheme 51. Application of compound 105 in the total synthesis of maremycin B. This figure is used with permission from the Royal
Society of Chemistry [124] .
Scheme 52. Spirolactonization reaction of isatins for construction of chiral spirolactones.
Scheme 53. Spirolactonization reaction of isatins 106 with 3-alkylidene oxindoles 107. This figure is used with permission from the
Royal Society of Chemistry [128] .
With the help of alkaloid-squaramide bifunctional organocatalyst C28, the cyclization was initiated with the
adol reaction between activated intermediate s-cis enolate A and 106 led to the formation of intermediate
B. Subsequent intramolecular lactonization, leading to the cleavage of the oxindole ring and generation of a
broad range of enantioenriched spirocyclic oxindole-pentenelactone derivatives 108 in good to excellent
yields with high enantioselectivities [Scheme 53].