Page 77 - Read Online
P. 77
Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38 Page 41 of 54
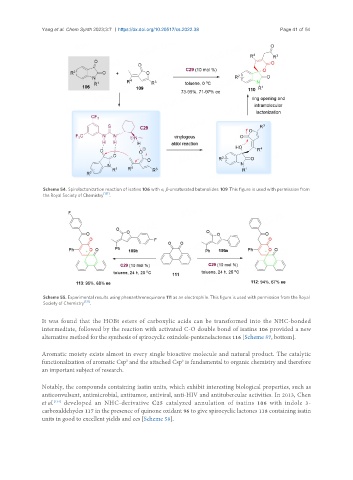
Scheme 54. Spirolactonization reaction of isatins 106 with α, β-unsaturated butenolides 109. This figure is used with permission from
[131]
the Royal Society of Chemistry .
Scheme 55. Experimental results using phenanthrenequinone 111 as an electrophile. This figure is used with permission from the Royal
Society of Chemistry [131] .
It was found that the HOBt esters of carboxylic acids can be transformed into the NHC-bonded
intermediate, followed by the reaction with activated C-O double bond of isatins 106 provided a new
alternative method for the synthesis of spirocyclic oxindole-pentenelactones 116 [Scheme 57, bottom].
Aromatic moiety exists almost in every single bioactive molecule and natural product. The catalytic
functionalization of aromatic Csp and the attached Csp is fundamental to organic chemistry and therefore
3
2
an important subject of research.
Notably, the compounds containing isatin units, which exhibit interesting biological properties, such as
anticonvulsant, antimicrobial, antitumor, antiviral, anti-HIV and antitubercular activities. In 2013, Chen
et al. developed an NHC-derivative C25 catalyzed annulation of isatins 106 with indole 3-
[134]
carboxaldehydes 117 in the presence of quinone oxidant 96 to give spirocyclic lactones 118 containing isatin
units in good to excellent yields and ees [Scheme 58].