Page 73 - Read Online
P. 73
Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38 Page 37 of 54
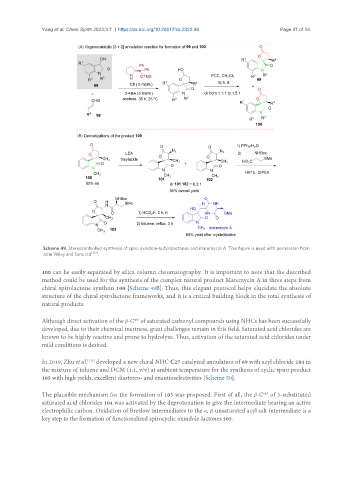
Scheme 49. Stereocontrolled synthesis of spiro oxindole-butyrolactones and maremycin A. This figure is used with permission from
John Wiley and Sons Ltd [123] .
100 can be easily separated by silica column chromatography. It is important to note that the described
method could be used for the synthesis of the complex natural product Maremycin A in three steps from
chiral spirolactone synthon 100 [Scheme 49B]. Thus, this elegant protocol helps elucidate the absolute
structure of the chiral spirolactone frameworks, and it is a critical building block in the total synthesis of
natural products.
sp3
Although direct activation of the β-C of saturated carbonyl compounds using NHCs has been successfully
developed, due to their chemical inertness, great challenges remain in this field. Saturated acid chlorides are
known to be highly reactive and prone to hydrolyze. Thus, activation of the saturated acid chlorides under
mild conditions is desired.
In 2019, Zhu et al. developed a new chiral NHC C27 catalyzed annulation of 69 with acyl chloride 104 in
[124]
the mixture of toluene and DCM (1:1, v/v) at ambient temperature for the synthesis of cyclic spiro product
105 with high yields, excellent diastereo- and enantioselectivities [Scheme 50].
The plausible mechanism for the formation of 105 was proposed. First of all, the β-C of 3-substituted
sp3
saturated acid chlorides 104 was activated by the deprotonation to give the intermediate bearing an active
electrophilic carbon. Oxidation of Breslow intermediates to the α, β-unsaturated acyl salt intermediate is a
key step to the formation of functionalized spirocyclic oxindole-lactones 105.