Page 79 - Read Online
P. 79
Yang et al. Chem Synth 2023;3:7 https://dx.doi.org/10.20517/cs.2022.38 Page 43 of 54
Scheme 57. Spirolactonization reaction of isatins 106 and HOBt esters of carboxylic acids 115. This figure is used with permission from
the American Chemical Society [133] .
spirocyclic oxindole-butyrolactones 122 were obtained in good yield with good diastereo- and
[137]
enantioselectivities [Scheme 60] .
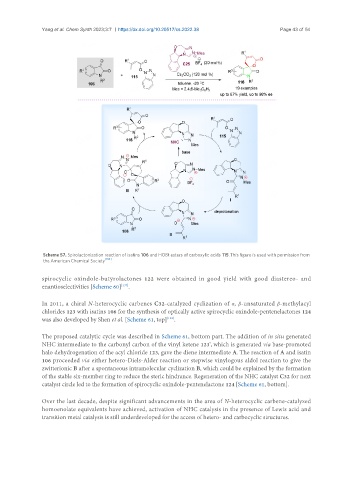
In 2011, a chiral N-heterocyclic carbenes C32-catalyzed cyclization of α, β-unsaturated β-methylacyl
chlorides 123 with isatins 106 for the synthesis of optically active spirocyclic oxindole-pentenelactones 124
was also developed by Shen et al. [Scheme 61, top] .
[138]
The proposed catalytic cycle was described in Scheme 61, bottom part. The addition of in situ generated
NHC intermediate to the carbonyl carbon of the vinyl ketene 123', which is generated via base-promoted
halo dehydrogenation of the acyl chloride 123, gave the diene intermediate A. The reaction of A and isatin
106 proceeded via either hetero-Diels-Alder reaction or stepwise vinylogous aldol reaction to give the
zwitterionic B after a spontaneous intramolecular cyclization B, which could be explained by the formation
of the stable six-member ring to reduce the steric hindrance. Regeneration of the NHC catalyst C32 for next
catalyst circle led to the formation of spirocyclic oxindole-pentenelactone 124 [Scheme 61, bottom].
Over the last decade, despite significant advancements in the area of N-heterocyclic carbene-catalyzed
homoenolate equivalents have achieved, activation of NHC catalysis in the presence of Lewis acid and
transition metal catalysis is still underdeveloped for the access of hetero- and carbocyclic structures.