Page 66 - Read Online
P. 66
Gurska et al. Cancer Drug Resist 2023;6:674-87 https://dx.doi.org/10.20517/cdr.2023.39 Page 682
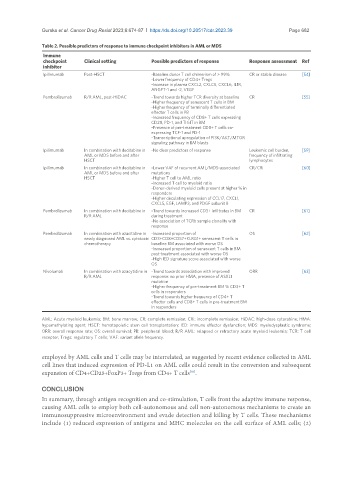
Table 2. Possible predictors of response to immune checkpoint inhibitors in AML or MDS
Immune
checkpoint Clinical setting Possible predictors of response Response assessment Ref
inhibitor
Ipilimumab Post-HSCT -Baseline donor T cell chimerism of > 99% CR or stable disease [54]
-Lower frequency of CD4+ Tregs
-Increase in plasma CXCL2, CXLC5, CXCL6, IL1R,
ANGPT-1 and -2, VEGF
Pembrolizumab R/R AML, post-HiDAC -Trend towards higher TCR diversity at baseline CR [55]
-Higher frequency of senescent T cells in BM
-Higher frequency of terminally differentiated
effector T cells in PB
-Increased frequency of CD8+ T cells expressing
CD28, PD-1, and TIGIT in BM
-Presence of pre-treatment CD8+ T cells co-
expressing TCF-1 and PD-1
-Transcriptional upregulation of PI3K/AKT/MTOR
signaling pathway in BM blasts
Ipilimumab In combination with decitabine in -No clear predictors of response Leukemic cell burden, [59]
AML or MDS before and after frequency of infiltrating
HSCT lymphocytes
Ipilimumab In combination with decitabine in -Lower VAF of recurrent AML/MDS-associated CR/CRi [60]
AML or MDS before and after mutations
HSCT -Higher T cell to AML ratio
-Increased T cell to myeloid ratio
-Donor-derived myeloid cells present at higher % in
responders
-Higher circulating expression of CCL17, CXCL1,
CXCL5, EGF, LAMP3, and PDGF subunit B
Pembrolizumab In combination with decitabine in -Trend towards increased CD3+ infiltrates in BM CR [61]
R/R AML during treatment
-No association of TCRb sample clonality with
response
Pembrolizumab In combination with azacitidine in -Increased proportion of OS [62]
newly diagnosed AML vs. cytotoxic CD3+CD8+CD57+KLRG1+ senescent T cells in
chemotherapy baseline BM associated with worse OS
-Increased proportion of senescent T cells in BM
post-treatment associated with worse OS
-High IED signature score associated with worse
OS
Nivolumab In combination with azacytidine in -Trend towards association with improved ORR [63]
R/R AML response: no prior HMA, presence of ASXL1
mutation
-Higher frequency of pre-treatment BM % CD3+ T
cells in responders
-Trend towards higher frequency of CD4+ T
effector cells and CD8+ T cells in pre-treatment BM
in responders
AML: Acute myeloid leukemia; BM: bone marrow; CR: complete remission; CRi: incomplete remission; HiDAC: high-dose cytarabine; HMA:
hypomethylating agent; HSCT: hematopoietic stem cell transplantation; IED: immune effector dysfunction; MDS: myelodysplastic syndrome;
ORR: overall response rate; OS: overall survival; PB: peripheral blood; R/R AML: relapsed or refractory acute myeloid leukemia; TCR: T cell
receptor; Tregs: regulatory T cells; VAF: variant allele frequency.
employed by AML cells and T cells may be interrelated, as suggested by recent evidence collected in AML
cell lines that induced expression of PD-L1 on AML cells could result in the conversion and subsequent
expansion of CD4+CD25+FoxP3+ Tregs from CD4+ T cells .
[66]
CONCLUSION
In summary, through antigen recognition and co-stimulation, T cells front the adaptive immune response,
causing AML cells to employ both cell-autonomous and cell non-autonomous mechanisms to create an
immunosuppressive microenvironment and evade detection and killing by T cells. These mechanisms
include (1) reduced expression of antigens and MHC molecules on the cell surface of AML cells; (2)