Page 65 - Read Online
P. 65
Page 681 Gurska et al. Cancer Drug Resist 2023;6:674-87 https://dx.doi.org/10.20517/cdr.2023.39
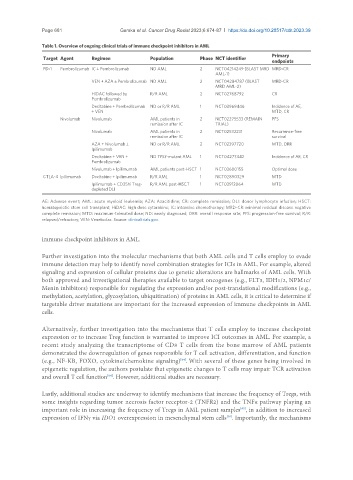
Table 1. Overview of ongoing clinical trials of immune checkpoint inhibitors in AML
Primary
Target Agent Regimen Population Phase NCT identifier
endpoints
PD-1 Pembrolizumab IC ± Pembrolizumab ND AML 2 NCT04214249 (BLAST MRD MRD-CR
AML-1)
VEN + AZA ± Pembrolizumab ND AML 2 NCT04284787 (BLAST MRD-CR
MRD AML-2)
HiDAC followed by R/R AML 2 NCT02768792 CR
Pembrolizumab
Decitabine + Pembrolizumab ND or R/R AML 1 NCT03969446 Incidence of AE,
± VEN MTD, CR
Nivolumab Nivolumab AML patients in 2 NCT02275533 (REMAIN PFS
remission after IC TRIAL)
Nivolumab AML patients in 2 NCT02532231 Recurrence-free
remission after IC survival
AZA + Nivolumab ± ND or R/R AML 2 NCT02397720 MTD, ORR
Ipilimumab
Decitabine + VEN + ND TP53-mutant AML 1 NCT04277442 Incidence of AE, CR
Pembrolizumab
Nivolumab ± Ipilimumab AML patients post-HSCT 1 NCT03600155 Optimal dose
CTLA-4 Ipilimumab Decitabine + Ipilimumab R/R AML 1 NCT02890329 MTD
Ipilimumab + CD25hi Treg- R/R AML post-HSCT 1 NCT03912064 MTD
depleted DLI
AE: Adverse event; AML: acute myeloid leukemia; AZA: Azacitidine; CR: complete remission; DLI: donor lymphocyte infusion; HSCT:
hematopoietic stem cell transplant; HiDAC: high dose cytarabine; IC: intensive chemotherapy; MRD-CR: minimal residual disease negative
complete remission; MTD: maximum-tolerated dose; ND: newly diagnosed; ORR: overall response rate; PFS: progression-free survival; R/R:
relapsed/refractory; VEN: Venetoclax. Source: clinicaltrials.gov.
immune checkpoint inhibitors in AML.
Further investigation into the molecular mechanisms that both AML cells and T cells employ to evade
immune detection may help to identify novel combination strategies for ICIs in AML. For example, altered
signaling and expression of cellular proteins due to genetic alterations are hallmarks of AML cells. With
both approved and investigational therapies available to target oncogenes (e.g., FLT3, IDH1/2, NPM1c/
Menin inhibitors) responsible for regulating the expression and/or post-translational modifications (e.g.,
methylation, acetylation, glycosylation, ubiquitination) of proteins in AML cells, it is critical to determine if
targetable driver mutations are important for the increased expression of immune checkpoints in AML
cells.
Alternatively, further investigation into the mechanisms that T cells employ to increase checkpoint
expression or to increase Treg function is warranted to improve ICI outcomes in AML. For example, a
recent study analyzing the transcriptome of CD8 T cells from the bone marrow of AML patients
demonstrated the downregulation of genes responsible for T cell activation, differentiation, and function
[64]
(e.g., NF-KB, FOXO, cytokine/chemokine signaling) . With several of these genes being involved in
epigenetic regulation, the authors postulate that epigenetic changes to T cells may impair TCR activation
[64]
and overall T cell function . However, additional studies are necessary.
Lastly, additional studies are underway to identify mechanisms that increase the frequency of Tregs, with
some insights regarding tumor necrosis factor receptor-2 (TNFR2) and the TNFα pathway playing an
important role in increasing the frequency of Tregs in AML patient samples , in addition to increased
[65]
expression of IFNγ via IDO1 overexpression in mesenchymal stem cells . Importantly, the mechanisms
[36]