Page 16 - Read Online
P. 16
Page 12 of 23 Skoreński et al. Rare Dis Orphan Drugs J 2023;2:6 https://dx.doi.org/10.20517/rdodj.2022.21
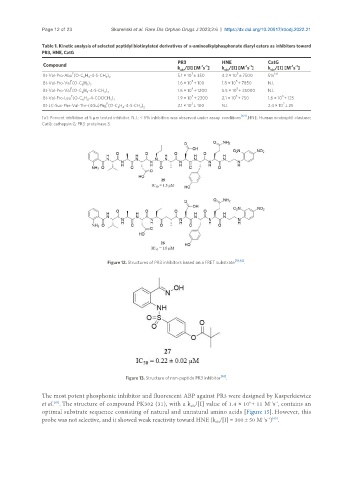
Table 1. Kinetic analysis of selected peptidyl biotinylated derivatives of α-aminoalkylphosphonate diaryl esters as inhibitors toward
PR3, HNE, CatG
PR3 HNE CatG
Compound -1 -1 -1 -1 -1 -1
k /[I] [M s ] k /[I] [M s ] k /[I] [M s ]
obs
obs
obs
P 3 5 [a]
Bt-Val-Pro-Abu (O-C H -4-S-CH ) 5.1 × 10 ± 350 4.2 × 10 ± 7500 5%
6 4 3 2
P 3 5
Bt-Val-Pro-Val (O-C H ) 1.6 × 10 ± 100 1.8 × 10 ± 7850 N.I.
6
5 2
4
P
5
Bt-Val-Pro-Val (O-C H -4-S-CH ) 1.6 × 10 ± 1200 5.5 × 10 ± 25000 N.I.
4
6
3 2
P 4 5 3
Bt-Val-Pro-Leu (O-C H -4-COOCH ) 1.9 × 10 ± 2300 2.1 × 10 ± 750 1.6 × 10 ± 125
6 4 3 2
P 3 2
Bt-LC-Suc-Phe-Val-Thr-(4Gu)Phg (O-C H -4-S-CH ) 2.1 × 10 ± 100 N.I. 2.4 × 10 ± 25
4
3 2
6
[a]: Percent inhibition at 5 µm tested inhibitor. N.I.: < 5% inhibition was observed under assay conditions [64] . HNE: Human neutrophil elastase;
CatG: cathepsin G; PR3: proteinase 3.
[58,62]
Figure 12. Structures of PR3 inhibitors based on a FRET substrate .
Figure 13. Structure of non-peptide PR3 inhibitor [63] .
The most potent phosphonic inhibitor and fluorescent ABP against PR3 were designed by Kasperkiewicz
[65]
6
-1 -1
et al. . The structure of compound PK302 (31), with a k /[I] value of 1.4 × 10 ± 11 M s , contains an
obs
optimal substrate sequence consisting of natural and unnatural amino acids [Figure 15]. However, this
probe was not selective, and it showed weak reactivity toward HNE (k /[I] = 300 ± 50 M s ) .
-1 -1 [65]
obs