Page 17 - Read Online
P. 17
Skoreński et al. Rare Dis Orphan Drugs J 2023;2:6 https://dx.doi.org/10.20517/rdodj.2022.21 Page 13 of 23
[60,61]
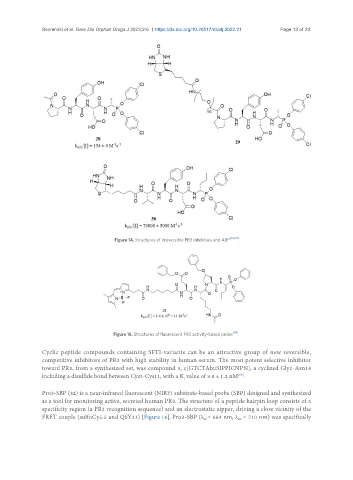
Figure 14. Structures of irreversible PR3 inhibitors and ABP .
Figure 15. Structures of fluorescent PR3 activity-based probe [65] .
Cyclic peptide compounds containing SFTI-variants can be an attractive group of new reversible,
competitive inhibitors of PR3 with high stability in human serum. The most potent selective inhibitor
toward PR3, from a synthesized set, was compound 3, c[GTCTAbuSIPPICNPN], a cyclized Gly1-Asn14
including a disulfide bond between Cys3-Cys11, with a K value of 9.8 ± 1.2 nM .
[66]
i
Pro3-SBP (32) is a near-infrared fluorescent (NIRF) substrate-based probe (SBP) designed and synthesized
as a tool for monitoring active, secreted human PR3. The structure of a peptide hairpin loop consists of a
specificity region (a PR3 recognition sequence) and an electrostatic zipper, driving a close vicinity of the
FRET couple (sulfoCy5.5 and QSY21) [Figure 16]. Pro3-SBP (λ = 684 nm, λ = 710 nm) was specifically
em
ex