Page 113 - Read Online
P. 113
Page 16 of 21 Sun et al. Microstructures 2023;3:2023032 https://dx.doi.org/10.20517/microstructures.2023.32
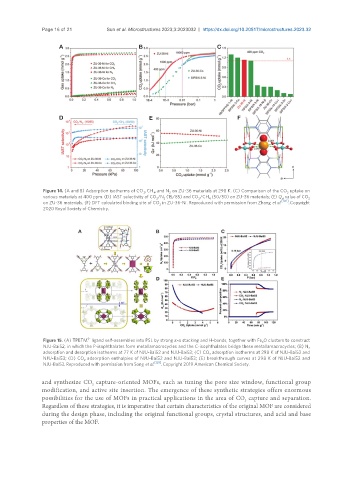
Figure 14. (A and B) Adsorption isotherms of CO , CH , and N on ZU-36 materials at 298 K. (C) Comparison of the CO uptake on
4
2
2
2
various materials at 400 ppm. (D) IAST selectivity of CO /N (15/85) and CO /CH (50/50) on ZU-36 materials; (E) Q value of CO 2
2
2
st
2
4
on ZU-36 materials. (F) DFT calculated binding site of CO in ZU-36-Ni. Reproduced with permission from Zhang et al. [102] . Copyright
2
2020 Royal Society of Chemistry.
6-
Figure 15. (A) TPBTM ligand self-assembles into PSL by strong π-π stacking and H-bonds, together with Fe O clusters to construct
3
NJU-Bai52, in which the P-isophthalates form metallamacrocycles and the C-isophthalates bridge these metallamacrocycles; (B) N
2
adsorption and desorption isotherms at 77 K of NJU-Bai52 and NJU-Bai53; (C) CO adsorption isotherms at 298 K of NJU-Bai52 and
2
adsorption enthalpies of NJU-Bai52 and NJU-Bai53; (E) breakthrough curves at 298 K of NJU-Bai52 and
NJU-Bai53; (D) CO 2
NJU-Bai53. Reproduced with permission from Song et al. [128] . Copyright 2019 American Chemical Society.
and synthesize CO capture-oriented MOFs, such as tuning the pore size window, functional group
2
modification, and active site insertion. The emergence of these synthetic strategies offers enormous
possibilities for the use of MOFs in practical applications in the area of CO capture and separation.
2
Regardless of these strategies, it is imperative that certain characteristics of the original MOF are considered
during the design phase, including the original functional groups, crystal structures, and acid and base
properties of the MOF.