Page 110 - Read Online
P. 110
Sun et al. Microstructures 2023;3:2023032 https://dx.doi.org/10.20517/microstructures.2023.32 Page 13 of 21
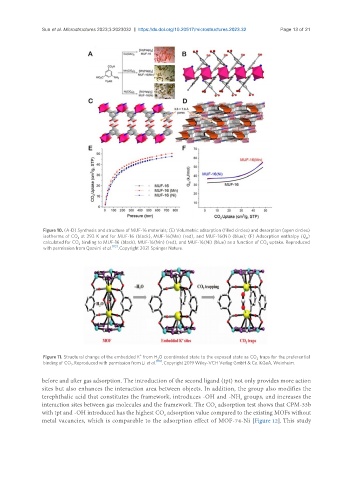
Figure 10. (A-D) Synthesis and structure of MUF-16 materials; (E) Volumetric adsorption (filled circles) and desorption (open circles)
isotherms of CO at 293 K and for MUF-16 (black), MUF-16(Mn) (red), and MUF-16(Ni) (blue); (F) Adsorption enthalpy (Q )
2 st
calculated for CO binding to MUF-16 (black), MUF-16(Mn) (red), and MUF-16(Ni) (blue) as a function of CO uptake. Reproduced
2 2
with permission from Qazvini et al. [113] . Copyright 2021 Springer Nature.
+
Figure 11. Structural change of the embedded K from H O coordinated state to the exposed state as CO traps for the preferential
2 2
binding of CO . Reproduced with permission from Li et al. [119] . Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
2
before and after gas adsorption. The introduction of the second ligand (tpt) not only provides more action
sites but also enhances the interaction area between objects. In addition, the group also modifies the
terephthalic acid that constitutes the framework, introduces -OH and -NH groups, and increases the
2
interaction sites between gas molecules and the framework. The CO adsorption test shows that CPM-33b
2
with tpt and -OH introduced has the highest CO adsorption value compared to the existing MOFs without
2
metal vacancies, which is comparable to the adsorption effect of MOF-74-Ni [Figure 12]. This study