Page 107 - Read Online
P. 107
Page 10 of 21 Sun et al. Microstructures 2023;3:2023032 https://dx.doi.org/10.20517/microstructures.2023.32
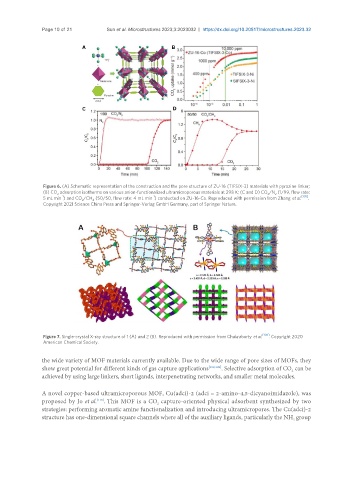
Figure 6. (A) Schematic representation of the construction and the pore structure of ZU-16 (TIFSIX-3) materials with pyrazine linker;
(B) CO adsorption isotherms on various anion-functionalized ultramicroporous materials at 298 K; (C and D) CO /N (1/99, flow rate:
2
2
2
-1
-1
5 mL min ) and CO /CH (50/50, flow rate: 4 mL min ) conducted on ZU-16-Co. Reproduced with permission from Zhang et al. [105] .
4
2
Copyright 2021 Science China Press and Springer-Verlag GmbH Germany, part of Springer Nature.
Figure 7. Single-crystal X-ray structure of 1 (A) and 2 (B). Reproduced with permission from Chakraborty et al. [106] . Copyright 2020
American Chemical Society.
the wide variety of MOF materials currently available. Due to the wide range of pore sizes of MOFs, they
show great potential for different kinds of gas capture applications [108,109] . Selective adsorption of CO can be
2
achieved by using large linkers, short ligands, interpenetrating networks, and smaller metal molecules.
A novel copper-based ultramicroporous MOF, Cu(adci)-2 (adci = 2-amino-4,5-dicyanoimidazole), was
proposed by Jo et al. . This MOF is a CO capture-oriented physical adsorbent synthesized by two
[110]
2
strategies: performing aromatic amine functionalization and introducing ultramicropores. The Cu(adci)-2
structure has one-dimensional square channels where all of the auxiliary ligands, particularly the NH group
2