Page 103 - Read Online
P. 103
Page 6 of 21 Sun et al. Microstructures 2023;3:2023032 https://dx.doi.org/10.20517/microstructures.2023.32
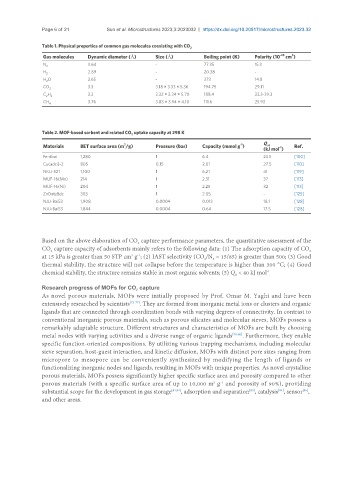
Table 1. Physical properties of common gas molecules coexisting with CO 2
-25
3
Gas molecules Dynamic diameter (Å) Size (Å) Boiling point (K) Polarity (10 cm )
N 2 3.64 - 77.35 15.3
H 2.89 - 20.38 -
2
H O 2.65 - 373 14.8
2
CO 3.3 3.18 × 3.33 × 5.36 194.75 29.11
2
C H 2 3.3 3.32 × 3.34 × 5.70 188.4 33.3-39.3
2
CH 4 3.76 3.83 × 3.94 × 4.10 111.6 25.93
Table 2. MOF-based sorbent and related CO uptake capacity at 298 K
2
-1
2
st
Materials BET surface area (m /g) Pressure (bar) Capacity (mmol g ) Q -1 Ref.
(kJ mol )
Fe-dbai 1,280 1 6.4 23.5 [100]
Cu(adci)-2 805 0.15 2.01 27.5 [110]
NKU-521 1,100 1 6.21 41 [119]
MUF-16(Mn) 214 1 2.31 37 [113]
MUF-16(Ni) 204 1 2.25 32 [113]
ZnDatzBdc 303 1 2.05 - [125]
NJU-Bai52 1,908 0.0004 0.013 18.1 [128]
NJU-Bai53 1,844 0.0004 0.64 17.5 [128]
Based on the above elaboration of CO capture performance parameters, the quantitative assessment of the
2
CO capture capacity of adsorbents mainly refers to the following data: (1) The adsorption capacity of CO
2
2
at 15 kPa is greater than 50 STP cm g ; (2) IAST selectivity (CO /N = 15/85) is greater than 500; (3) Good
3
-1
2
2
thermal stability, the structure will not collapse before the temperature is higher than 300 °C; (4) Good
chemical stability, the structure remains stable in most organic solvents; (5) Q < 40 kJ mol -1 .
st
Research progress of MOFs for CO capture
2
As novel porous materials, MOFs were initially proposed by Prof. Omar M. Yaghi and have been
extensively researched by scientists [72-77] . They are formed from inorganic metal ions or clusters and organic
ligands that are connected through coordination bonds with varying degrees of connectivity. In contrast to
conventional inorganic porous materials, such as porous silicates and molecular sieves, MOFs possess a
remarkably adaptable structure. Different structures and characteristics of MOFs are built by choosing
metal nodes with varying activities and a diverse range of organic ligands [78-80] . Furthermore, they enable
specific function-oriented compositions. By utilizing various trapping mechanisms, including molecular
sieve separation, host-guest interaction, and kinetic diffusion, MOFs with distinct pore sizes ranging from
micropore to mesopore can be conveniently synthesized by modifying the length of ligands or
functionalizing inorganic nodes and ligands, resulting in MOFs with unique properties. As novel crystalline
porous materials, MOFs possess significantly higher specific surface area and porosity compared to other
-1
porous materials (with a specific surface area of up to 10,000 m g and porosity of 90%), providing
2
substantial scope for the development in gas storage [81,82] , adsorption and separation , catalysis , sensor ,
[85]
[83]
[84]
and other areas.