Page 98 - Read Online
P. 98
Page 6 of 9 Zhao et al. Microstructures 2023;3:2023022 https://dx.doi.org/10.20517/microstructures.2022.46
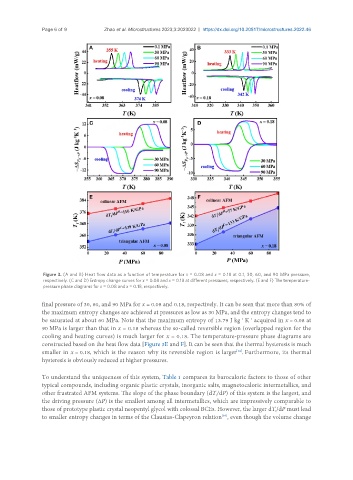
Figure 3. (A and B) Heat flow data as a function of temperature for x = 0.08 and x = 0.18 at 0.1, 30, 60, and 90 MPa pressure,
respectively. (C and D) Entropy change curves for x = 0.08 and x = 0.18 at different pressures, respectively. (E and F) The temperature-
pressure phase diagrams for x = 0.08 and x = 0.18, respectively.
final pressure of 30, 60, and 90 MPa for x = 0.08 and 0.18, respectively. It can be seen that more than 80% of
the maximum entropy changes are achieved at pressures as low as 30 MPa, and the entropy changes tend to
be saturated at about 60 MPa. Note that the maximum entropy of 13.79 J kg K acquired in x = 0.08 at
-1
-1
90 MPa is larger than that in x = 0.18 whereas the so-called reversible region (overlapped region for the
cooling and heating curves) is much larger for x = 0.18. The temperature-pressure phase diagrams are
constructed based on the heat flow data [Figure 3E and F]. It can be seen that the thermal hysteresis is much
smaller in x = 0.18, which is the reason why its reversible region is larger . Furthermore, its thermal
[14]
hysteresis is obviously reduced at higher pressures.
To understand the uniqueness of this system, Table 1 compares its barocaloric factors to those of other
typical compounds, including organic plastic crystals, inorganic salts, magnetocaloric intermetallics, and
other frustrated AFM systems. The slope of the phase boundary (dT/dP) of this system is the largest, and
t
the driving pressure (ΔP) is the smallest among all intermetallics, which are impressively comparable to
those of prototype plastic crystal neopentyl glycol with colossal BCEs. However, the larger dT/dP must lead
t
to smaller entropy changes in terms of the Clausius-Clapeyron relation , even though the volume change
[39]